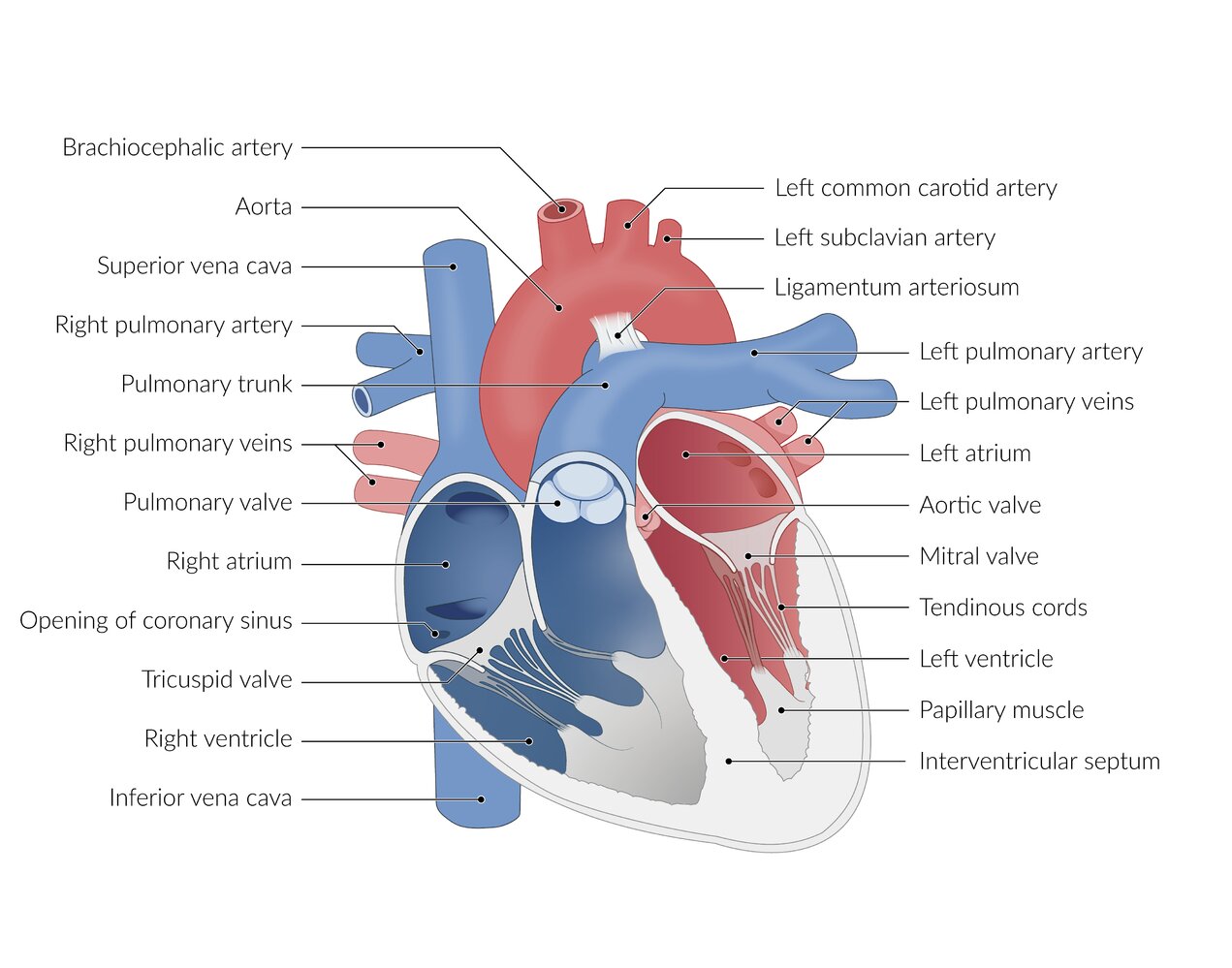
Acyanotic congenital heart defects (ACHDs) are cardiac malformations that affect the atrial or ventricular walls, heart valves, or large blood vessels. Common causes include genetic defects (e.g., trisomies), maternal infections (e.g., rubella), and maternal use of drugs or alcohol during pregnancy. ACHDs are characterized by a left-to-right shunt, which causes pulmonary hypertension and right atrial and ventricular hypertrophy. Symptoms depend on the extent of the malformation and the resulting impairment of cardiac function. Infants may be asymptomatic or present with signs of respiratory distress, failure to thrive, and/or symptoms of heart failure. Characteristic heart murmurs are important clues for establishing the diagnosis, which is typically confirmed by visualizing the defect on transthoracic echocardiography (TTE). Further studies (e.g., chest x-ray, MRI, cardiac CT, or cardiac catheterization) may be required for surgical evaluation and planning. Surgical or transcatheter repair is indicated in selected patients and pharmacological treatment is required to manage complications, e.g., heart failure, arrhythmias, and Eisenmenger syndrome.
Common acyanotic CHDs
| Overview of acyanotic CHDs [1][2][3] | |||
|---|---|---|---|
| Description | Associated conditions and risk factors | Management | |
| Atrial septal defect (ASD) |
|
|
|
| Ventricular septal defect (VSD) [8] |
|
|
|
| Atrioventricular septal defect (AVSD) |
|
|
|
| Patent foramen ovale (PFO) [11] |
|
|
|
| Patent ductus arteriosus (PDA) [12][13] |
|
|
|
| Coarctation of the aorta [15] |
|
|
|
| Pulmonary valve stenosis [17][18] |
|
|
|
The “3 Ds” of ACHDs (in order of frequency): VSD, PDA, ASD.
Pathogenesis [19]
Principles
- Congenital heart defects (CHDs) are caused by the disruption of the normal sequence of cardiac morphogenesis.
- CHDs may lead to the formation of pathological connections (shunts) between the right and left heart chambers, allowing blood to flow along the pressure gradient from high pressure to low pressure.
- The shunts are classified according to the direction of the blood flow as either left-to-right or right-to-left.
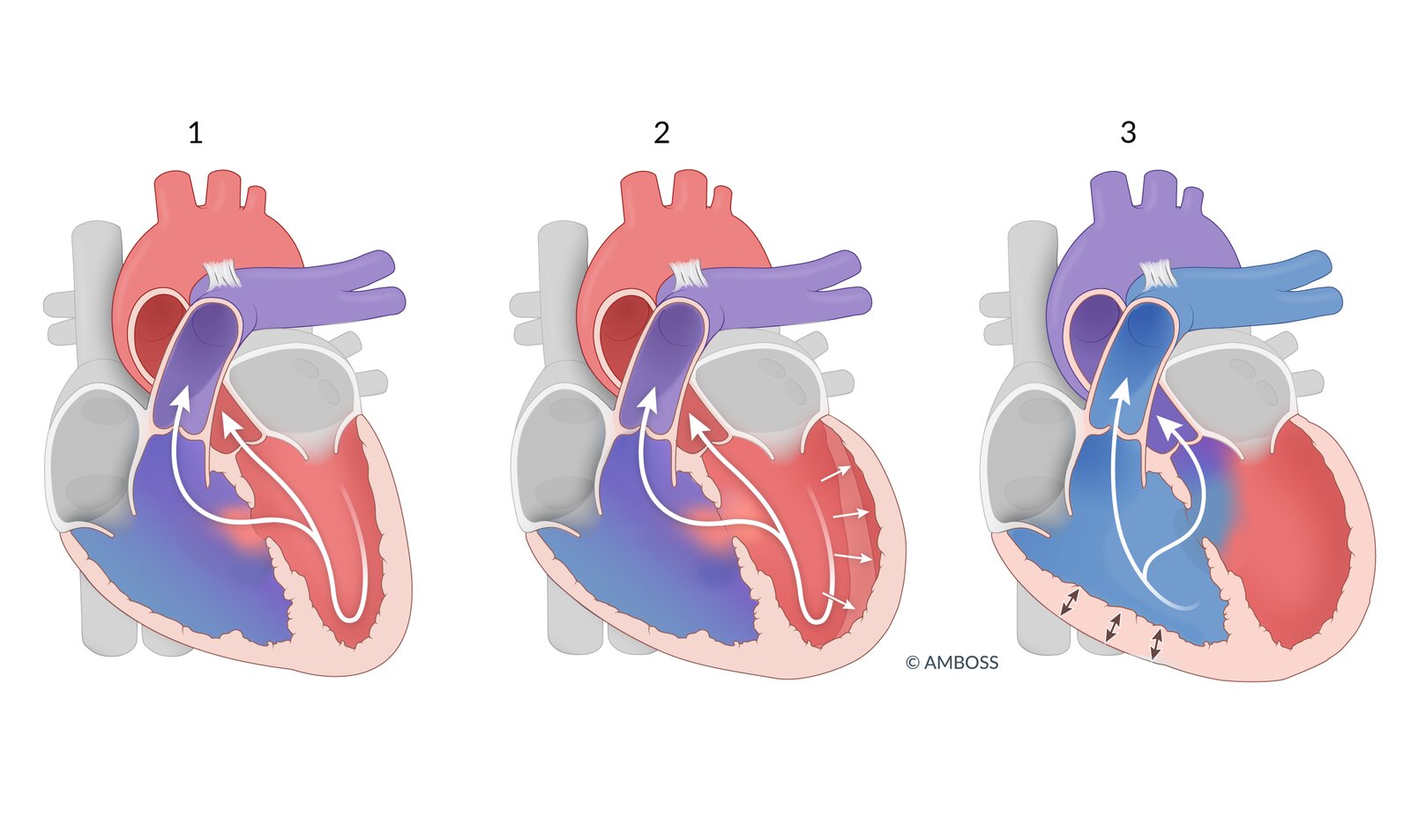
Shunt types
-
Left-to-right shunt
- Oxygenated blood from the lungs is shunted back into the pulmonary circulation via an atrial septal defect (ASD), ventricular septal defect (VSD), or patent ductus arteriosus (PDA) → pulmonary hypertension
- Right ventricular pressure overload → right-sided heart hypertrophy (cardiomegaly on x-ray) and heart failure but no cyanosis
- Right-to-left shunt: blood flows from the right to the left heart via a shunt → deoxygenated blood entering the systemic circulation → cyanosis
- See “Eisenmenger syndrome” for details on how a left-to-right shunt can develop into a right-to-left shunt over time.
Left-to-Right shunts = LateR cyanosis. Right-to-Left shunts = eaRLy cyanosis.
General clinical features
For specific features, see “Clinical features” in the respective subsections.
Nonspecific findings
- Failure to thrive
- Recurrent bronchopulmonary infections
- Normal skin tone
-
Exercise intolerance
- Fatigue, pallor, and diaphoresis (sweating)
- Tachycardia
- Dyspnea
- Grunting, nasal flaring, retractions, and/or head bobbing may be seen [20]
Heart failure
-
Right heart failure
- Hepatic venous congestion with hepatomegaly
- Peripheral edema is rarely seen in infants. [21]
-
Left heart failure
- Tachypnea, pulmonary edema
- Low cardiac output: ↓ blood pressure, pallor, sweating, cool extremities, syncope
Medical management of ACHDs [3][10]
Consult a cardiologist specializing in ACHDs for all patients; life-long close follow-up is required.
- General measures
- Nutritional support as needed
- Information on the immunization schedule
- Counseling on contraception as required
- Encourage regular exercise; request cardiopulmonary exercise testing in patients with moderate to severe symptoms. [10]
- Ductal-dependent CHDs: prostaglandin E1 infusion to prevent PDA closure
- Provide heart failure management.
- Respiratory support as needed
- Diuretics to decrease fluid volume
- ACE inhibitors to lower systemic vascular resistance
- Inotropic agents (e.g., digoxin) to improve cardiac contractility
- Determine the need for surgical repair and postoperative antibiotic prophylaxis.
- Assess for and manage Eisenmenger syndrome.
Most patients with ACHDs can participate in regular moderate physical activity. [10]
| Comparison of common features of VSD and ASD [3] | |||
|---|---|---|---|
| Ventricular septum defect (VSD) [8] | Atrial septum defect (ASD) | ||
| Epidemiology |
|
|
|
| Etiology |
|
||
|
|
||
| Clinical features | Small defect |
|
|
| Medium-sized or large defect |
|
|
|
| Auscultation |
|
|
|
| ECG | Small defect |
|
|
| Medium-sized or large defect |
|
||
| Echocardiography |
|
|
|
| Chest x-ray |
|
|
|
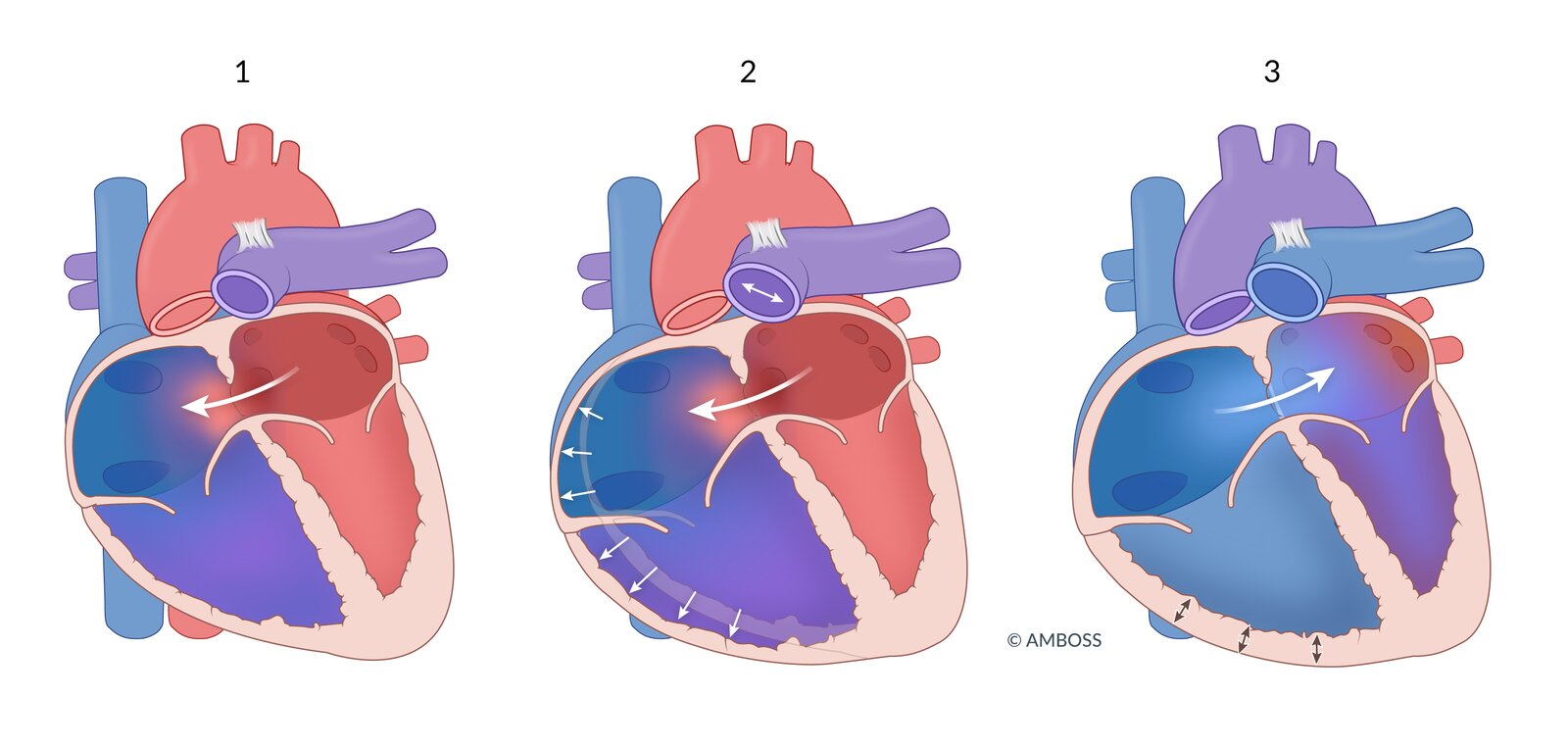
Description
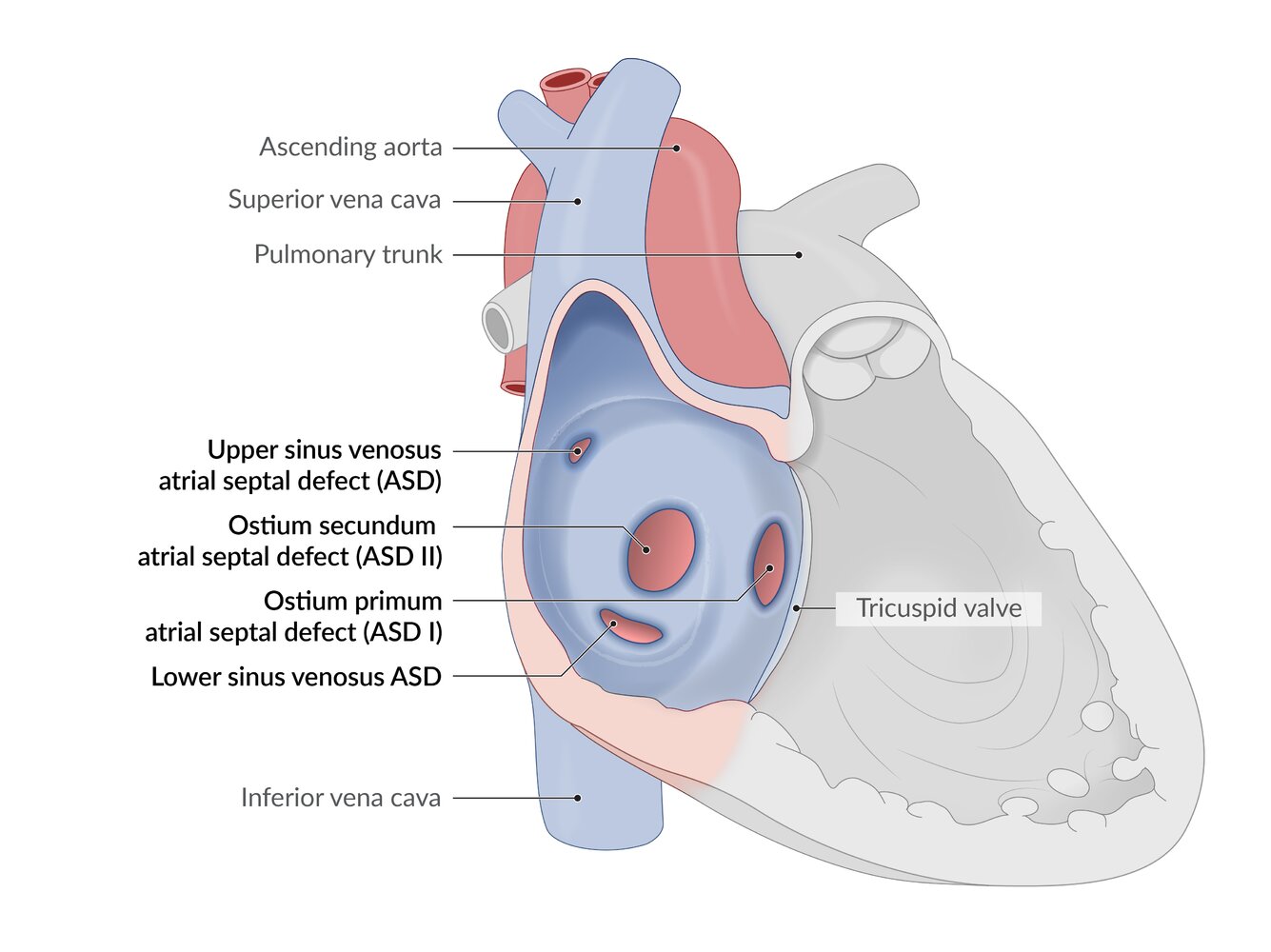
- A defect in the atrial wall that may result from impaired growth or excessive resorption of the atrial septum.
Epidemiology [3]
- Third most common CHD (∼ 1/2,000live births) [22]
- Sex: ♀ > ♂
Etiology
- Down syndrome [4]
- Fetal alcohol syndrome [5]
- Intrauterine infections (e.g., TORCH) [6][7]
-
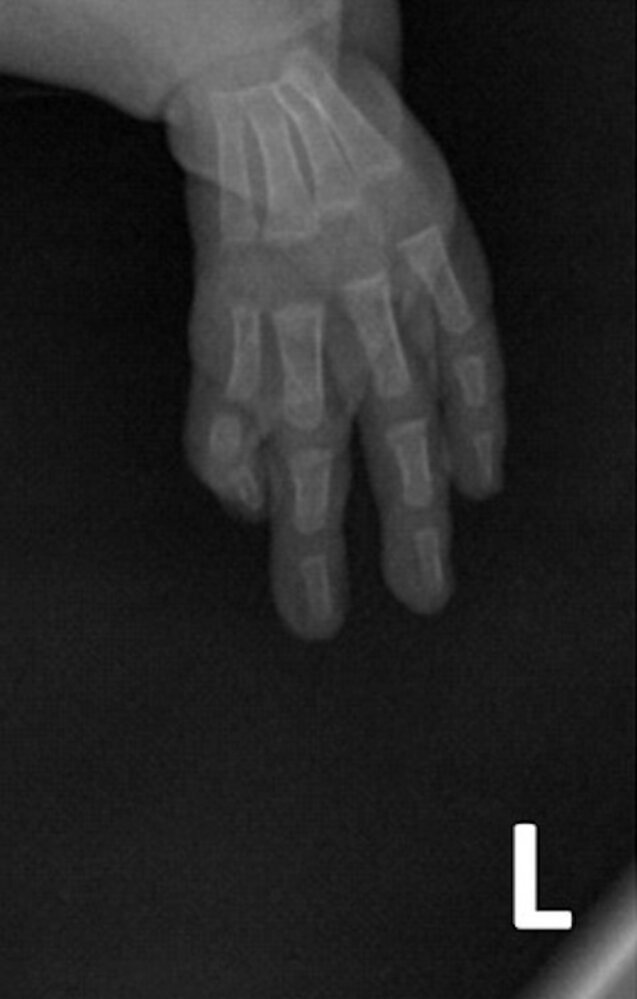
Holt-Oram syndrome (hand-heart syndrome) [3]
- Autosomal dominant disorder
- Affects ∼ 1/100,000 children
- Characterized by ASD, a first-degree heart block, and abnormalities of the upper limbs (e.g., absent radial bones)
Pathophysiology
-
Impaired growth or excessive resorption of the atrial septa in utero leads to atrial septal defects (absent atrial septa tissue).
- Ostium primum atrial septal defect (ASD I): ∼ 15–20% (usually accompanied by other heart defects)
- Ostium secundum atrial septal defect (ASD II): ∼ 70% (usually isolated)
- Typically a low-pressure, low-volume, minor left-to-right shunt (therefore, patients are usually asymptomatic)
- ASD → oxygenated blood shunting from LA to RA → ↑ O2 saturation in the RA → ↑ O2 saturation in RV and pulmonary artery
- In more severe defects, the shunts may lead to supraventricular arrhythmias, pulmonary hypertension, and/or Eisenmenger syndrome.


Clinical features [3]
- Small defects: usually asymptomatic
- Medium to large defects
- Clinical presentation varies from asymptomatic to overt heart failure.
- Symptoms typically manifest with advancing age (∼ 30–40 years of age). [23]
- Clinical features include: [3][23]
- Exertional dyspnea
- Fatigue
- Recurrent respiratory infections
- Palpitations (e.g., due to supraventricular arrhythmias)
- Syncope
- Symptoms of heart failure (e.g., peripheral edema)
- Stroke or TIA (e.g., from paradoxical embolism)
- See “General clinical features” in “Overview.”
Auscultation [3]
- Mid-systolic ejectionmurmur over the second left ICS, parasternal
- Widely split second heart sound (S2) over the second left ICS,; which is fixed(does not change with respiration)
- Soft mid-diastolicmurmur over the lower left sternal border
Diagnostics [10][23][24]
-
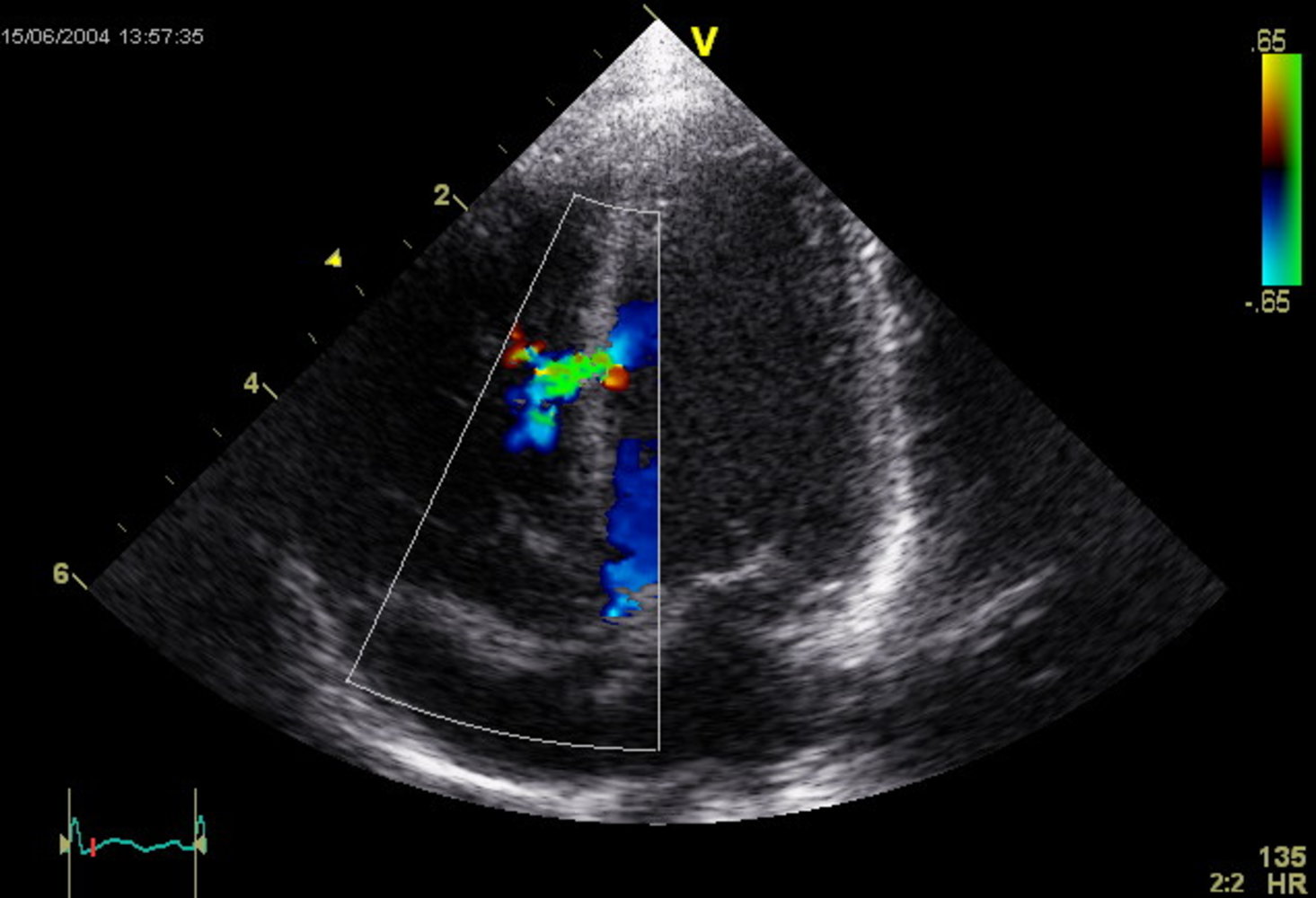
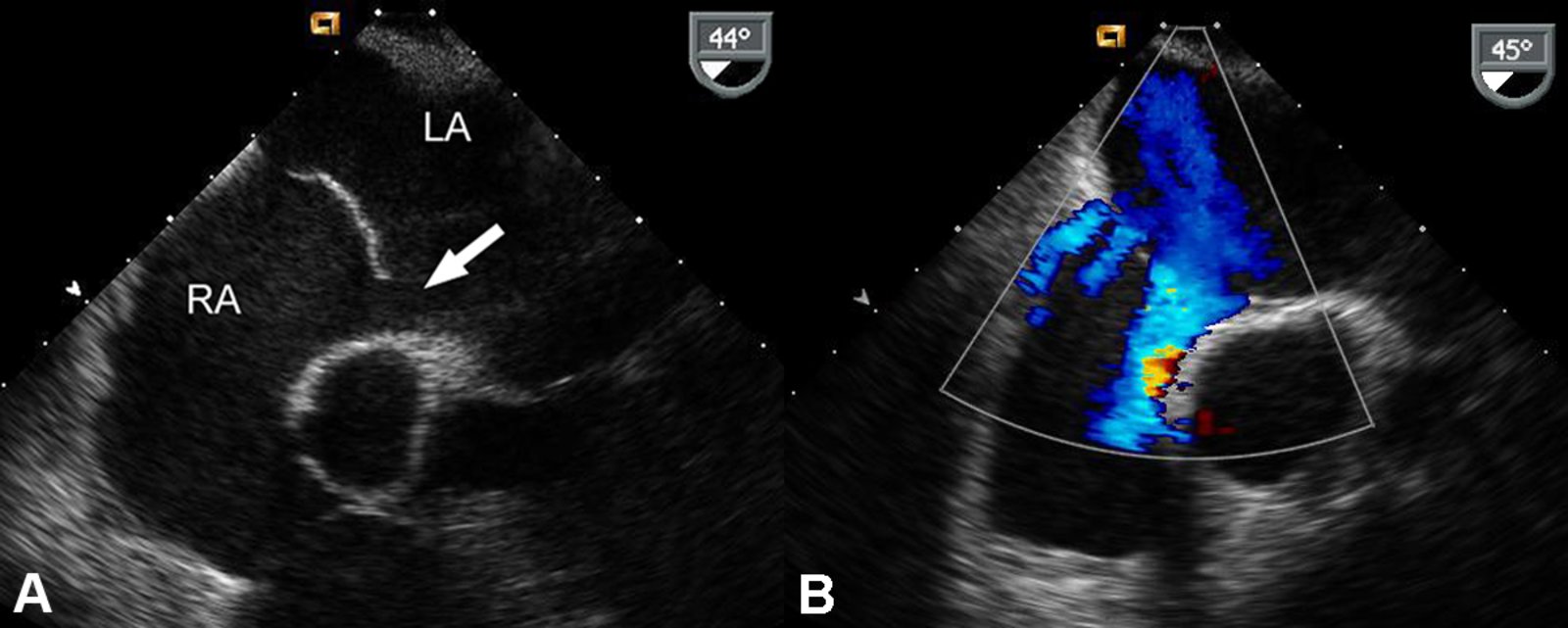
Echocardiography (confirmatory test) :
-
TTE with Doppler
- Confirms interatrial communication
- Best visualized in the apical four-chamber and subcostal views
- Agitated saline study: if TTE findings are equivocal [23]
-
TTE with Doppler
-
ECG
-
Signs of RV hypertrophy, e.g.:
- Vertical or right axis deviation
- P pulmonale, and/or PR prolongation
- Complete or incomplete right bundle branch block
- Atrial tachyarrhythmias, e.g., atrial fibrillation, atrial flutter, are common in adults with ASDs. [23]
-
Signs of RV hypertrophy, e.g.:
-
Chest x-ray
- Rounding of the left heart contour due to an enlarged right atrium, right ventricle, and pulmonary artery
- Enlarged lung markings due to enhanced pulmonary vasculature
-
Pulse oximetry
- Perform at rest and during exercise, especially for patients with moderate to severe symptoms. [10]
- Helps to determine the magnitude and direction of the shunt

Additional testing
-
Other noninvasive imaging
-
Cardiac MRI or CT [10]
- Consider preprocedurally for anatomical guidance. [10]
- Qp:Qs ratio can be calculated with cardiac MRI
- CT coronary angiography: alternative to cardiac catheterization in patients at low or intermediate risk of CAD
-
Cardiac MRI or CT [10]
-
Cardiac catheterization
- Reserved for patients with risk factors for CAD
- Most accurate method to determine detailed hemodynamics, e.g.:
- Shunt magnitude
- Pulmonary artery pressure
- Qp:Qs ratio
Management [3][10]
General principles
- Management should be guided by a cardiologist specializing in congenital heart disease.
- Manage associated conditions and complications (e.g., atrial fibrillation, tachyarrhythmias, pulmonary hypertension).
- Small and/or asymptomatic ASDs: follow-up with physical examination and serial echocardiography
- Larger or symptomatic ASDs may require surgical management.
Surgical management
-
Indications
- Large left-to-right shunts (e.g., Qp:Qs ratio ≥ 1.5:1 without pulmonary hypertension)
- Right atrial or ventricular hypertrophy
- Symptoms of heart failure
- History of paradoxical embolism
- Platypnea-orthodeoxia syndrome
- Contraindication: patients with severe pulmonary hypertension [25]
-
Surgical repair methods
- Transcatheter closure
- Patch
- Open approach
-
Postrepair follow-up includes:
- Echocardiography
- Endocarditis prophylaxis
- Monitoring for complications (e.g., new chest pain, syncope, symptoms of arrhythmias)
Up to 40% of ASDs spontaneously close by 5 years of age. [26]
Surgical repair is contraindicated in patients with right-to-left shunts (e.g., in Eisenmenger syndrome). [10]
Complications [27]
- Paradoxical embolism (↑ risk of ischemic stroke): an embolus from the venous circulation passes from the right atrium through the ASD into the left atrium, enters the arterial circulation into the brain, and causes a stroke
- Heart failure
Description
- An abnormal communication between the left and right ventricle that results in left-to-right shunting
Epidemiology
- Most common congenital heart defect (∼ 4/1000live births) [10][28]
- Occurs as an isolated heart defect or in combination with others (e.g., AVSD, tetralogy of Fallot, TGA)
Etiology
- Genetic syndromes [3]
- Most commonly: Down syndrome, Edward syndrome, Patau syndrome
- Less commonly: Cri-du-chat syndrome, Apert syndrome
- Intrauterine infections (e.g., TORCH) [6][7]
- Maternal risk factors: diabetes, obesity, smoking [9][29]
- Acquired (rare): post-MI, aortic valve replacement [30][31]
Pathophysiology
- Most commonly located in the membranous part of the ventricular septum (pars membranacea)
-
Defect in ventricular septum →left-to-right shunt with the following consequences:
- RV volume overload →RV eccentric hypertrophy
- Excessive pulmonary blood flow → ↑ pulmonary arterypressure →pulmonary hypertension
- ↓ Cardiac output
- LV volume overload → LV eccentric hypertrophy
- ↑ O2 saturation in right ventricle and pulmonary artery

Clinical features [3][8]
General
- Small defects: usually asymptomatic
-
Medium or large defects
- Lead to heart failure within the first few weeks to months of life
- Become symptomatic after high pulmonary vascular resistance (PVR) present at birth starts to decrease: ↓ PVR →↓ right ventricular pressure →↑ left-to-rightshunt → symptoms
- See “Nonspecific findings” and “Heart failure” in “Overview” above.
- Hyperdynamic precordium may be detected in hemodynamically relevant defects.
Auscultation
-
Harsh holosystolic murmurover the left lower sternal border
- Becomes more intense with maneuvers that increase left ventricularafterload (e.g., handgrip)
- Typically louder in small defects
- Systolic thrill in the 3rd or 4th left ICS
- Mid-diastolic murmur over cardiac apex
- Loud pulmonic S2 (if pulmonary hypertension develops)

Symptoms of heart failure in children with VSD only develop when PVR decreases to adult levels and thus allows left-to-right shunting to occur.
Diagnostics [8][10][28]
-
Echocardiography: (confirmatory test): TTE is preferred over TEE. [28]
- To assess defect size, shunt volume, Qp:Qs ratio, and associated conditions, e.g., ↑ pulmonary artery pressure, outflow obstructions
- Doppler echocardiography is especially helpful for visualizing small VSDs.
-
ECG
- Small defects: normal ECG
-
Medium or large defects
- Signs of left atrial enlargement, e.g., P mitrale
-
Signs of LV hypertrophy due to volume loading, e.g.:
- ↑ QRS amplitude
- Left axis deviation
- Left atrial enlargement
-
Signs of RV hypertrophy if pulmonary hypertension or obstruction of the pulmonary outflow tract is present:
- Vertical or right axis deviation
- P pulmonale, and/or PR prolongation,
- Complete or incomplete right bundle branch block
-
Chest x-ray
- Small defects: normal chest x-ray
-
Medium or large defects
- Enhanced pulmonary vascular markings
- Rounding of the apex and left cardiac contour due to left atrial and ventricular hypertrophy
- Rounding of the mid-left heart border due to right ventricular hypertrophy and enlarged pulmonary artery (in later stages; due to ↑ pulmonary vascular resistance)

Additional testing
-
Other noninvasive imaging [10]
- Cardiac CT or MRI: Consider preprocedurally for anatomical guidance.
- CT coronary angiography: alternative to cardiac catheterization in patients with a low or intermediate risk of CAD
- Cardiac catheterization: Consider if there are equivocal findings on TTE, risk factors for CAD, or pulmonary vascular disease is suspected. [10][28]
Management [3][8][10][28]
Asymptomatic and small defects
- Spontaneous closure is common; surgical intervention is rarely required.
- Follow-up echocardiography is recommended.
Symptomatic and/or large defects
- Medical management of ACHDs: required for all patients.
-
Surgical repair: e.g., patch [32];
-
Indications
- Infants with large left-to-right shunts and clinical symptoms (e.g., failure to thrive, treatment-resistant congestive heart failure)
- Asymptomatic older children with large left-to-right shunts or evidence of left atrial or left ventricular enlargement
- Pulmonary hypertension
- Qp:Qs ratio ≥ 1.5:1 if pulmonary hypertension is not present [10]
- VSD with aortic insufficiency or aortic valve prolapse into the VSD
- Contraindicated in severe pulmonary hypertension [10]
-
Indications
-
Postrepair follow-up
- Clinical monitoring for the development of new symptoms
- Serial echocardiography to monitor for VSD patch leak
- Endocarditis prophylaxis for dental procedures for 6 months after transcatheter or surgical closure
VSD closure results in lower right ventricular and left atrial pressures and higher left ventricular pressures than preclosure values. [32]
VSD closure is contraindicated in patients with Eisenmenger syndrome. [3]
Complications
- Arrhythmias
- Heart failure
- Eisenmenger syndrome
- Infective endocarditis
- Aortic regurgitation
Definition [3]
A defect of atrioventricular valves (i.e., mitral and tricuspid valves) as well as the atrial and/or ventricular septum; previously referred to as endocardial cushion defects.
- Complete form: ASD and VSD, common AV valve
- Partial form: only ASD and minor AV valve abnormalities
Etiology
- Strongly associated with Down syndrome [33][34]
- Association with maternal diabetes and obesity has been shown in some studies. [35]
Pathophysiology [3]
- Complete form: (ASD and VSD) →atrial and ventricular left-to-rightshunt → excessive pulmonary blood flow and biventricular volume overload →pulmonary hypertension and heart failure
- Partial form (ASD only) → atrial left-to-right shunt → symptoms that may remain minimal until adulthood
- In both forms: abnormal AV valve → AV valve regurgitation → in utero heart failure (nonimmune hydrops fetalis) [36]
Clinical features [3]
- Complete form: See “Nonspecific findings” and “Heart failure” in “Overview” above.
- Partial form: See “Clinical features” in “Atrial septal defect (ASD).“
Diagnostics [37]
- Antenatal echocardiography: findings of endocardial cushion defect in first trimester →screening for Down syndrome
- Echocardiography (confirmatory test): to assess defect size, shunt volume, and global cardiac function
- ECG: left axis deviation due to LV hypertrophy
-
Chest x-ray
- Complete form: globalcardiomegaly, ↑ pulmonary markings
- Partial form: enlarged right heart and pulmonary artery
Treatment [3][10][37]
- All patients: Provide medical management of acyanotic CHDs as needed.
-
Surgical management: patch closure and AV valve reconstruction; generally indicated unless Eisenmenger syndrome has developed
- Complete form: generally between 3–6 months of age
- Partial form: generally between 2–4 years of age
- Older patients: elective procedure
Description
- A variant of cardiac anatomy in which the foramen ovale remains patent beyond 1 year of age
Epidemiology
- Prevalence: ∼ 25% of the general population [38]
Etiology
- Associated with Loeys-Dietz syndrome
Pathophysiology
- Failure of the atrial septum primum to fuse with the septum secundum following birth → persistence of foramen ovale; → mild left-to-right shunt
- A right-to-left shunt (i.e., shunt reversal) may be induced by certain maneuvers that increase right atrial pressure (e.g., Valsalva maneuver, coughing).
ASD = Septal tissue Deficiency. PFO = enough tissue, but Problems with Fusion.
Clinical features [39]
- Affected individuals are usually asymptomatic until complications due to right-to-left shunting occur; see “Complications.”
Diagnostics [39]
-
General principles
- Diagnosis of a PFO is frequently incidental (e.g., during PCI or cardiac surgery).
- Diagnostic studies are requested as a part of the assessment of conditions suggestive of PFO, e.g., cryptogenic stroke.
-
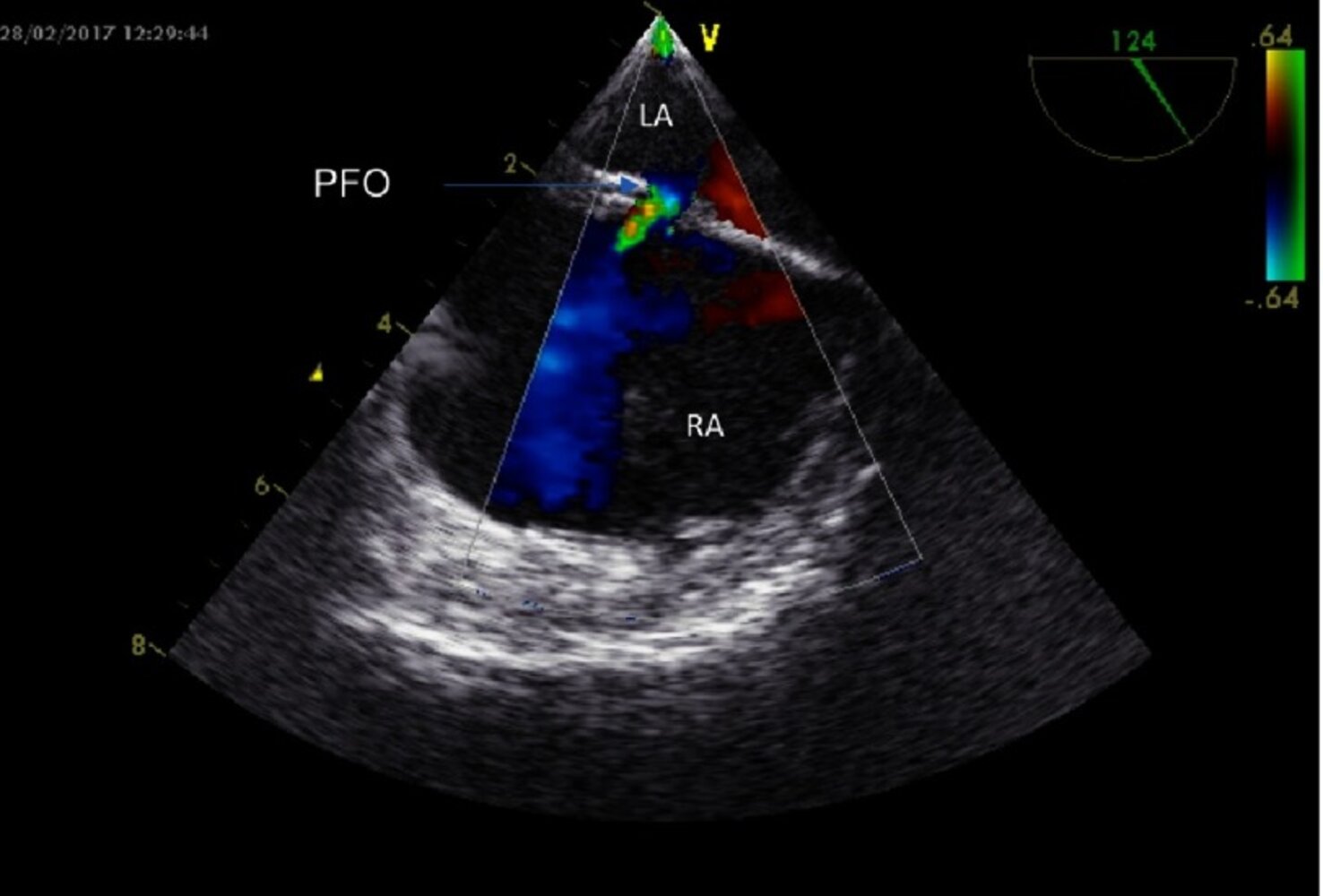
TTE with agitated saline (best initial study)
- Right-to-left shunt that increases with the Valsalva maneuver and coughing
- Other findings: atrial septal aneurysm [40]
-
TEE with agitated saline (most sensitive test) may be considered if:
- TTE is inconclusive
- There is a high index of suspicion for PFO despite negative TTE
- PFO anatomical assessment is needed after positive TTE
-
Transcranial Doppler with agitated saline
- Agitated saline is injected into a peripheral vein.
- The detection of microbubbles in a cerebral artery indicates a right-to-left shunt. [39]
Treatment [11][39]
- Asymptomatic PFO: Treatment is usually not required: (not associated with an increased risk of stroke). [39]
-
Confirmed PFO after an embolic event (e.g., ischemic stroke) [11]
- Multidisciplinary care by cardiology and neurology to determine the likelihood of paradoxical embolism
- Strategies to reduce subsequent stroke risk include:
- Antiplatelet agents or anticoagulation
- Surgical or percutaneous closure of the defect [11]
PFO is one of many possible causes of stroke. Reducing subsequent stroke risk should include the evaluation of other potential causes (e.g., arrhythmia, hypercoagulability, endocarditis). [11]
Complications
- Migraine with aura
- Ischemic stroke or TIA (often cryptogenic)
- Paradoxical embolism
- Systemic embolisms (e.g., renal infarction)
Description
- Failure of the ductus arteriosus to completely close postnatally
Epidemiology
- Incidence: 5–10% full-term births [41]
- In premature infants: 20–60% [41]
- Sex: ♀ > ♂ (2:1) [3]
Etiology
- Prematurity
- Maternal exposure during pregnancy
- Rubella infection (during the first trimester of pregnancy)
- Alcohol consumption
- Phenytoin use (fetal hydantoin syndrome)
- Prostaglandin use
- Respiratory distress syndrome
- Trisomies (e.g., Down syndrome)
Pathophysiology
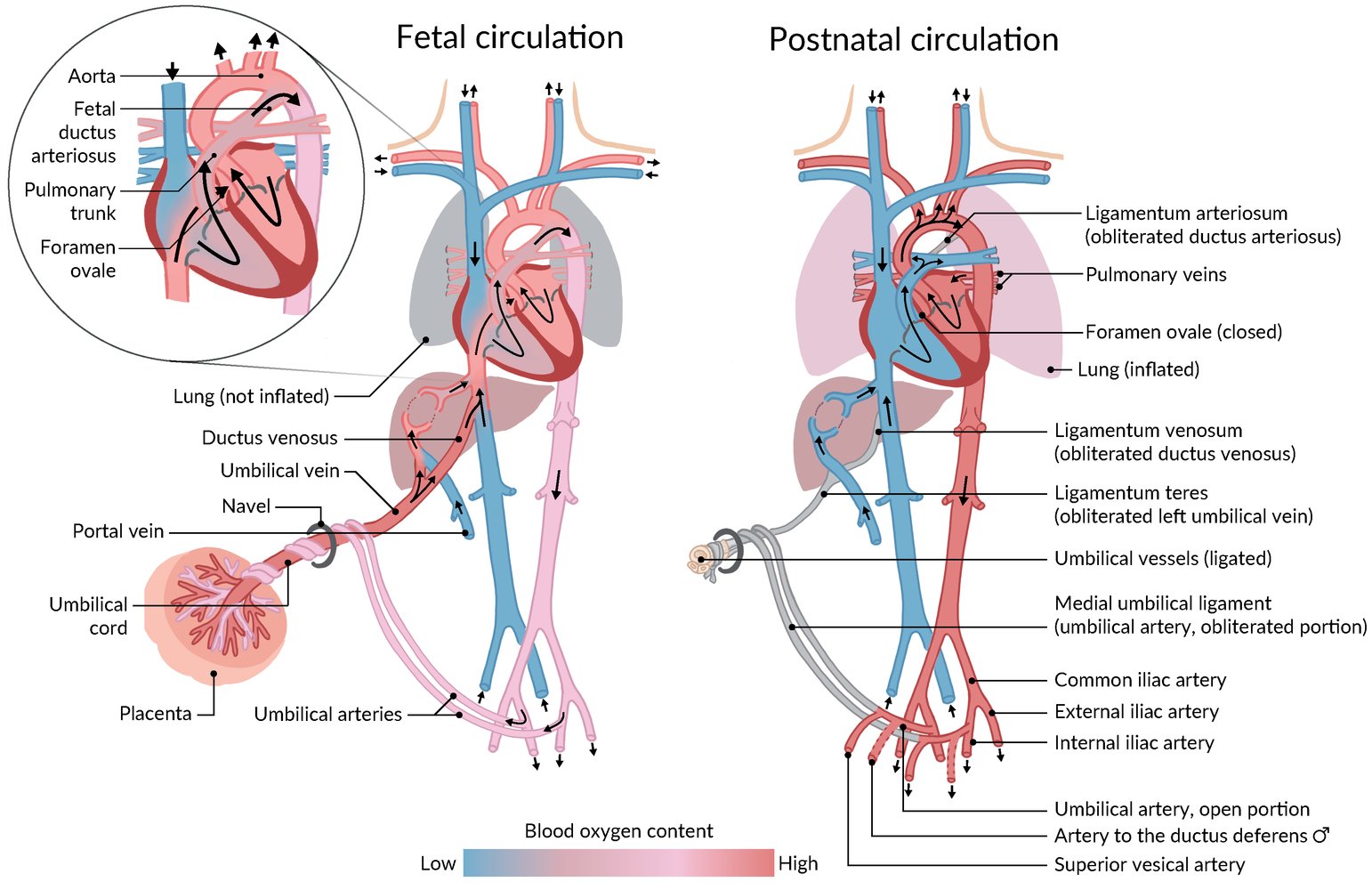
- Ductus arteriosus enables the underdeveloped lungs to be bypassed by the fetal circulation (normal right-to-left shunt) and remains patent in utero via PGE and low O2 tension.
- After birth, pulmonary vascular resistance decreases and thus allows for the reversal of the shunt from right-to-left to left-to-right.
- Failure of the ductus arteriosus to close after birth → persistent communication between the aorta and the pulmonary artery → left-to-right shunt → volume overload of the pulmonary vessels → continuous RV (and/or LV) strain → heart failure (see also “Overview” above)
- Eisenmenger syndrome may occur with shunt reversal and manifest with differential cyanosis.
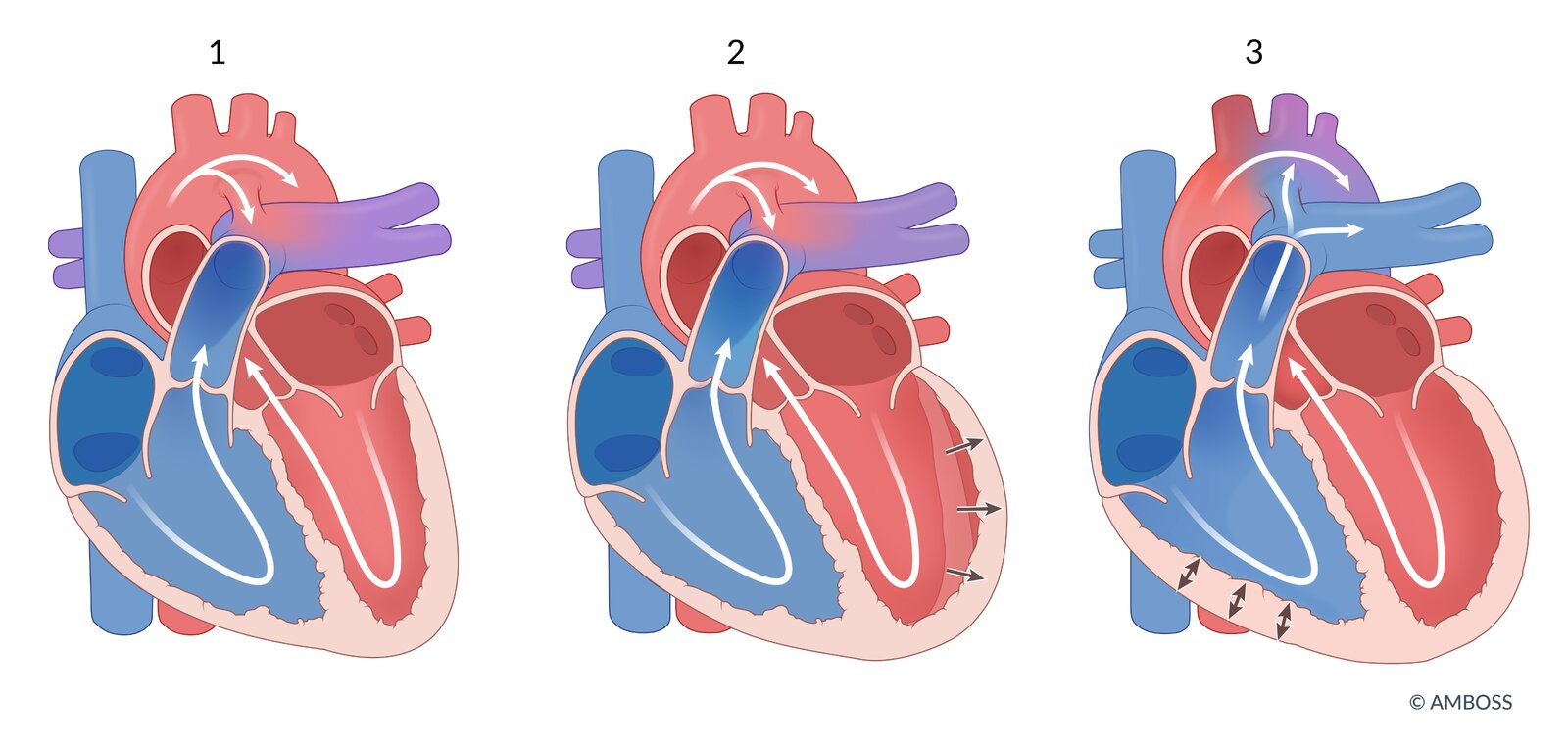
Clinical features
General
- Small PDA: asymptomatic with normal findings on physical examination
-
Large PDA
- Nonspecific symptoms (e.g., failure to thrive) and symptoms of heart failure in infancy (see the “Overview” above)
- Bounding peripheral pulses, wide pulse pressure [41]
- Heaving, laterally displaced apical impulse
Auscultation
- Small PDA: A murmur is sometimes heard incidentally during routine primary care visits.
- Large PDA: Machinery murmur: loud continuous murmur heard best in the left infraclavicular region; and loudest at S2
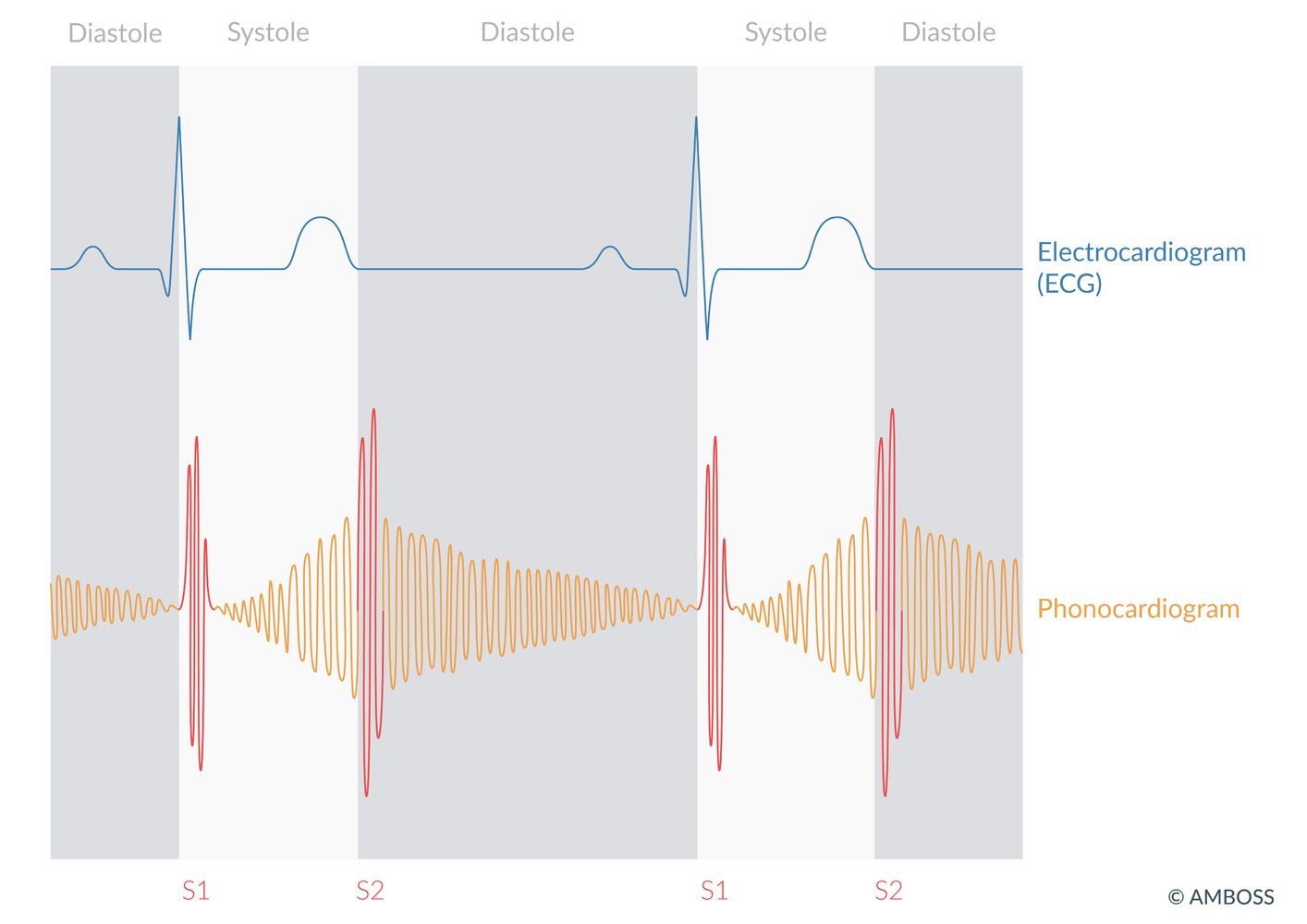
PDA comes with Prolonged Deafening Auscultation findings.
Diagnostics [10][12][42]
-
Echocardiography (confirmatory test for infants)
- Assesses shunt volume and pulmonary artery pressure
- Left atrial and ventricular hypertrophy may be seen.
- Color Doppler: may show blood flow from the aorta into the pulmonary artery
-
ECG
- Small PDA: normal ECG
-
Large PDA
- Left axis deviation due to LV hypertrophy
- May show RV hypertrophy if pulmonary hypertension has developed
-
Chest x-ray
- Prominent pulmonary artery and aortic knob at the upper left heart border
- Increased pulmonary markings
- Pulse oximetry: possible peripheral hypoxemia in the feet, especially with ambulation, if right-to-left shunting is present
Additional testing
- Other noninvasive imaging (e.g., cardiac CT, cardiac MRI): usually required for older children, adults, and adolescents to assess anatomy [43]
-
Cardiac catheterization
- To confirm hemodynamics and evaluate pulmonary vasoreactivity before repair or in complex CHDs [10]
- To assess for pulmonary hypertension
Management [12][13]
General principles
- Refer all patients to a congenital cardiac center for management.
- Management of PDA is complex and based on PDA (e.g., size of left-to-right shunt) and patient characteristics (e.g., weight, age).
- Assess all patients for comorbidities and complications (e.g., intestinal ischemia, Eisenmenger syndrome).
Pharmacological closure in premature infants [3]
Management of premature infants with a PDA requires a multidisciplinary team including a neonatal intensivist and pediatric cardiologist.
-
Indications
- Infants with birth weight < 1 kg requiring mechanical ventilation
- Infants with birth weight > 1 kg with symptomatic PDA (e.g., symptoms of heart failure, respiratory distress)
-
Contraindications
- Ductal-dependent CHD
- Persistent pulmonary hypertension of the newborn
- Oliguria
- Thrombocytopenia
- Recent hemorrhage (e.g., cerebral, intestinal, pulmonary)
- Necrotizing enterocolitis
- Treatment: Indomethacin and ibuprofen induce PDA closure by inhibiting prostaglandin synthesis. [3]
-
Alternatives
- Percutaneous catheter occlusion
- Surgical ligation
PDA closure is contraindicated if the PDA is required for survival, e.g., in ductal-dependent CHDs. Initiate prostaglandin E1 infusion to keep the ductus arteriosus patent until definitive treatment can be performed. [44]
Pharmacological closure is preferred for preterm infants as surgical closure is associated with increased morbidity and mortality and studies on transcatheter occlusion are lacking in these patients. [10][43][45]
Transcatheter and surgical closure [10][43]
Transcatheter or surgical closure is the treatment of choice for infants ≥ 6 kg, children, and adults.
-
Indications
- Symptoms of heart failure
- Failure to thrive
- Echocardiography showing left atrial and/or left ventricular enlargement with PA systolic pressure < 50% systemic and PVR < ⅓ systemic [43]
- Pulmonary hypertension without right-to-left shunt
-
Contraindications
- Ductal-dependent CHD
- Pulmonary hypertension with right-to-left shunt
-
Methods
- Transcatheter occlusion
- Surgical ligation
Complications [43]
- Heart failure in infancy
- Infective endocarditis
- Pulmonary hypertension and Eisenmenger syndrome in adolescents and adults (common)
- Differential cyanosis
Definition
- Narrowing of the aorta at the aortic isthmus or, rarely, in the descending thoracic or abdominal aorta
Epidemiology [46]
- Prevalence: 3/10,000 live births
- Sex: ♂ > ♀
Etiology [2]
Congenital
- The exact cause is unknown, but two hypotheses have been proposed:
- Hemodynamic: caused by underdevelopment of the aorta due to an abnormally decreased antegrade intrauterine blood flow
- Ductal: caused by closure of the ductus arteriosus tissue that extends into the thoracic aorta
- Associated with Turner syndrome (in 5–15% of female patients with coarctation) [16][47]
-
Often accompanied by a bicuspid aortic valve, VSD, and/or PDA
Acquired
- Takayasu arteritis
- Severe atherosclerosis
Pathophysiology [1][2]
-
Genetic defects and/or intrauterine ischemia →medial thickening and intimalhyperplasia → formation of a ridge encircling the aortic lumen →narrowing of the aorta →↑ flowproximalto the narrowing and↓ flowdistalto the narrowing
- Coarctation is most commonly juxtaductal.
- The coarctation most commonly occurs distal to the left subclavian artery, where the ductus arteriosus originates.
- Rarely, the coarctation occurs in the lower segments of the thoracic aorta or in the abdominal aorta
- In discrete coarctation: left ventricular outflow obstruction →myocardialhypertrophy and increased collateral blood flow (e.g., intercostal vessels, scapular vessels).
- In long-segment coarctation: closure of PDA after birth →left ventricular pressure and volume overload →hypoperfusion of organs and extremities distal to the stenosis [48]
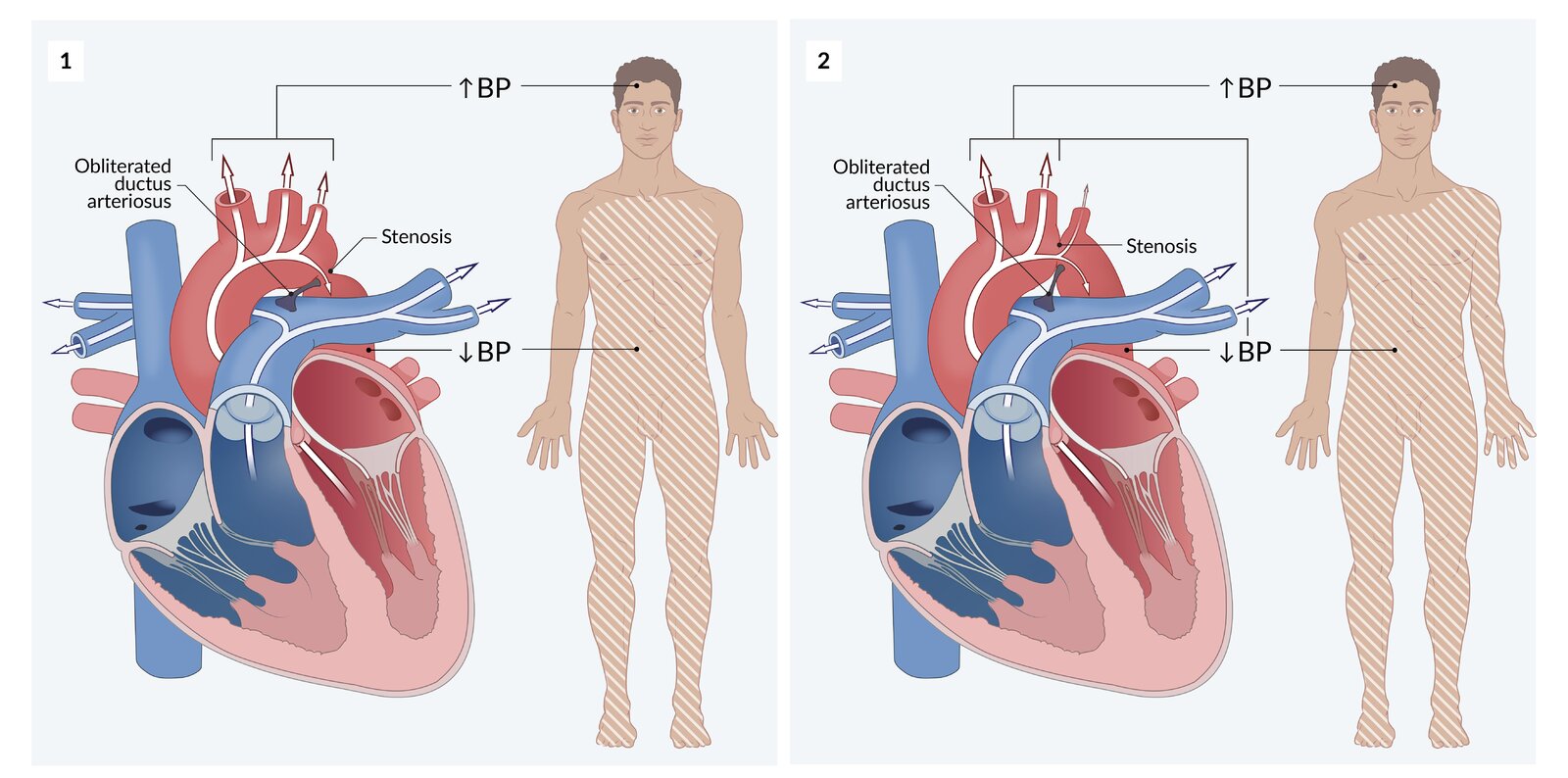
Clinical features [2]
General
-
Neonates
- Asymptomatic if the coarctation is mild and PDA is present
- Symptomatic in critical stenosis (blood flow to the lower body is PDA-dependent): See “Overview.”
-
Older infants, children, and adults may be asymptomatic. If present, symptoms include:
- Differential cyanosis: cyanosis of the lower extremities
- Brachial-femoral delay: weak femoral pulses
- ↑ Blood pressure (BP) in upper extremities and ↓ BP in lower extremities
- Cold feet and lower-extremityclaudication upon physical exertion
- Strong apical impulse displaced to the left
- Headache, epistaxis, tinnitus
- In severe stenosis: shock and multiorgan failure when ductus arteriosus closes
- In severe stenosis: See “Nonspecific findings” and “Heart failure” in “Overview” above.
Auscultation
- Systolic ejectionmurmur over left posterior hemithorax and/or continuous murmur in the left infraclavicular region and interscapular region
Diagnostics [3][10][49]
Evaluate for aortic coarctation in patients with hypertension and/or weakened femoral pulses, especially in younger patients.
-
Blood pressure measurements (best initial test): upper and lower extremities
- In distal narrowing of the left subclavian artery: ↑ BP in upper extremities and ↓ BP in lower extremities
- If the origin of the left subclavian artery is involved: BP in the right arm > BP in the left arm
- Ambulatory BP monitoring as part of the diagnosis and management of hypertension
- Pulse oximetry: postcoarctation ↓ SpO2 may be present
-
ECG
- Neonates: signs of RV hypertrophy
- Older children and adults: normal ECG or signs of LV hypertrophy on ECG
-
Chest x-ray
- ↑ Cardiothoracic ratio and pulmonary vascular markings
- Figure of 3 sign: dilation of the aorta and left subclavian artery cause an hourglass-like narrowing of the aorta and a 3-shaped outline, formed by an indentation at the site of coarctation and a post-stenotic dilatation in the descending aorta
-
Rib notching: a radiographic sign caused by collateral circulation between the internal thoracic and intercostal arteries (usually seen in patients > 5 years of age)
- Enlarged collateral vessels compress the neighboring ribs, causing pressure atrophy.
- Classically affects the inferior border of the 3rd–8th ribs
-
Doppler echocardiography (confirmatory test)
- To locate and assess the extent of stenosis
- To detect concurrent defects (e.g., VSD, PDA, bicuspid aortic valve) [49]
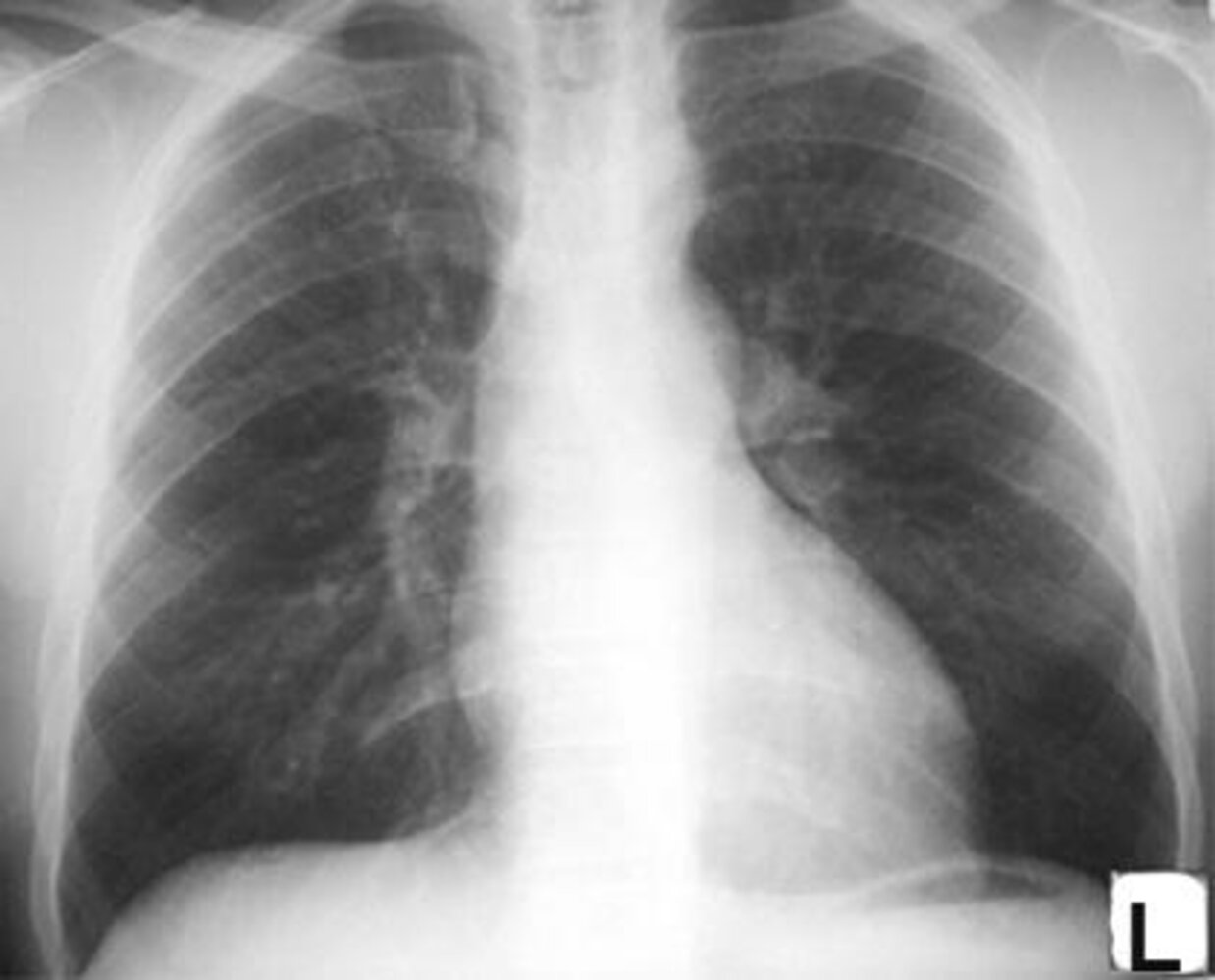
Additional testing
- Cardiopulmonary exercise testing: to evaluate for exercise-induced hypertension in adults [10]
-
MRI or CT (in adults) [10]
- CMR or CTA: to assess aortic anatomy and collateral circulation, and for intervention planning and follow-up
- MRA or CTA: may be considered to screen for intracranial aneurysms [10]
- Cardiac catheterization: for patients at risk for CAD before repair [49]
- Genetic testing: Consider testing for Turner syndrome if suspected based on other clinical findings. [16]
Treatment [10][15][49][50]
-
Neonates with critical coarctation
-
Initiate prostaglandin E1 infusion.
- Facilitates postcoarctation organ perfusion by maintaining the patency of the ductus arteriosus until surgical repair can be performed
- Example: alprostadil (off-label) [26][51]
- Medical management of ACHDs (e.g., inotropic support, respiratory support)
-
Initiate prostaglandin E1 infusion.
-
Noncritical coarctation [10][45];
- Stent placement
- Surgical correction (preferred method in neonates) [3]
- Balloon angioplasty in adults [10]
-
Medical management
- Management of hypertension for all patients [10]
- Follow-up to monitor for re-stenosis, aortic aneurysm, and aortic dissection
- Counsel on physical activity restriction for patients with hypertension or residual obstruction. [52]
Complications
- Secondary hypertension
- Aortic dissection and rupture
- Berry aneurysm leading to cerebral hemorrhage [53]
- Heart failure
- Infective endocarditis
- Postrepair recoarctation [10]
Description
- A valvular heart disease characterized by obstruction of blood outflow from the right ventricle into the pulmonary arteries during systole
Epidemiology
- Relatively common in the general population (∼10% of all CHDs) [3]
- Usually congenital (rarely acquired )
- Association with Noonan syndrome
Pathophysiology
- Pulmonary valve stenosis → right ventricular outflow obstruction → pressure overload → right ventricular hypertrophy
Clinical features
- Depending on the grade of stenosis, symptoms of heart failure may occur.
- Systolic murmur heard best over the second left ICS, parasternal
- S2 wide splitting
Diagnostics [10][17]
- Echocardiography (confirmatory test): helps to assess the severity of stenosis [10]
-
ECG [54]
- Normal in mild pulmonary stenosis
- Signs of RV hypertrophy in patients with severe stenosis
- Chest x-ray: may show pulmonary artery dilatation
- Cardiac catheterization: for patients at high risk of CAD before repair
Treatment [10][17]
-
Neonates with critical pulmonary valve stenosis [3][55]
- Maintain the PDA until definitive treatment can be performed.
- Method: prostaglandin E1 infusion, e.g., alprostadil (off-label) [26][51]
-
Noncritical pulmonary valve stenosis
- Indication: symptomatic patients (e.g., symptoms of heart failure, exercise intolerance) with moderate to severe pulmonary valve stenosis
- Procedures: balloon valvuloplasty of the pulmonary valve (preferred), pulmonary valve replacement
- Follow-up: postrepair pulmonary regurgitation monitoring
Description [3][56]
- A severe complication of ACHD in which the left-to-right shunt reverses over time due to pulmonary hypertension, resulting in cyanotic heart disease
- Can occur at any age, but usually develops during the late stages of ACHDs
Etiology [3]
Eisenmenger syndrome may develop with any cardiac defect with a left-to-right shunt; common defects include:
- Complete AVSD
- Persistent truncus arteriosus
- ASD
- VSD
- PDA
Pathogenesis [3][56]
- Left-to-right shunt→ prolongedpulmonary hypertension→ reactive constriction with permanent remodeling of pulmonary vessels → irreversible pulmonary hypertension
- Pulmonary hypertension → RV hypertrophy→ increased RV pressure
- RV pressure exceeds LV pressure→ shunt reversal (development of right-to-left shunt) → cyanosis; , digital clubbing, and polycythemia
Clinical features [3][57]
Eisenmenger syndrome develops gradually and children are often minimally symptomatic in the early stages; symptoms worsen with age and increasing pulmonary resistance.
-
Cyanosis
- Central cyanosis is prominent.
- Differential cyanosis: cyanosis in the lower extremities (seen in patients with PDA)
- Digital clubbing
- Dyspnea
- Chest pain
- Clinical features of heart failure
- Bleeding diathesis, e.g., epistaxis, easy bruising, hemoptysis, due to thrombocytopenia [10][57]
- Increased risk for thrombotic events due to hyperviscosity [57]
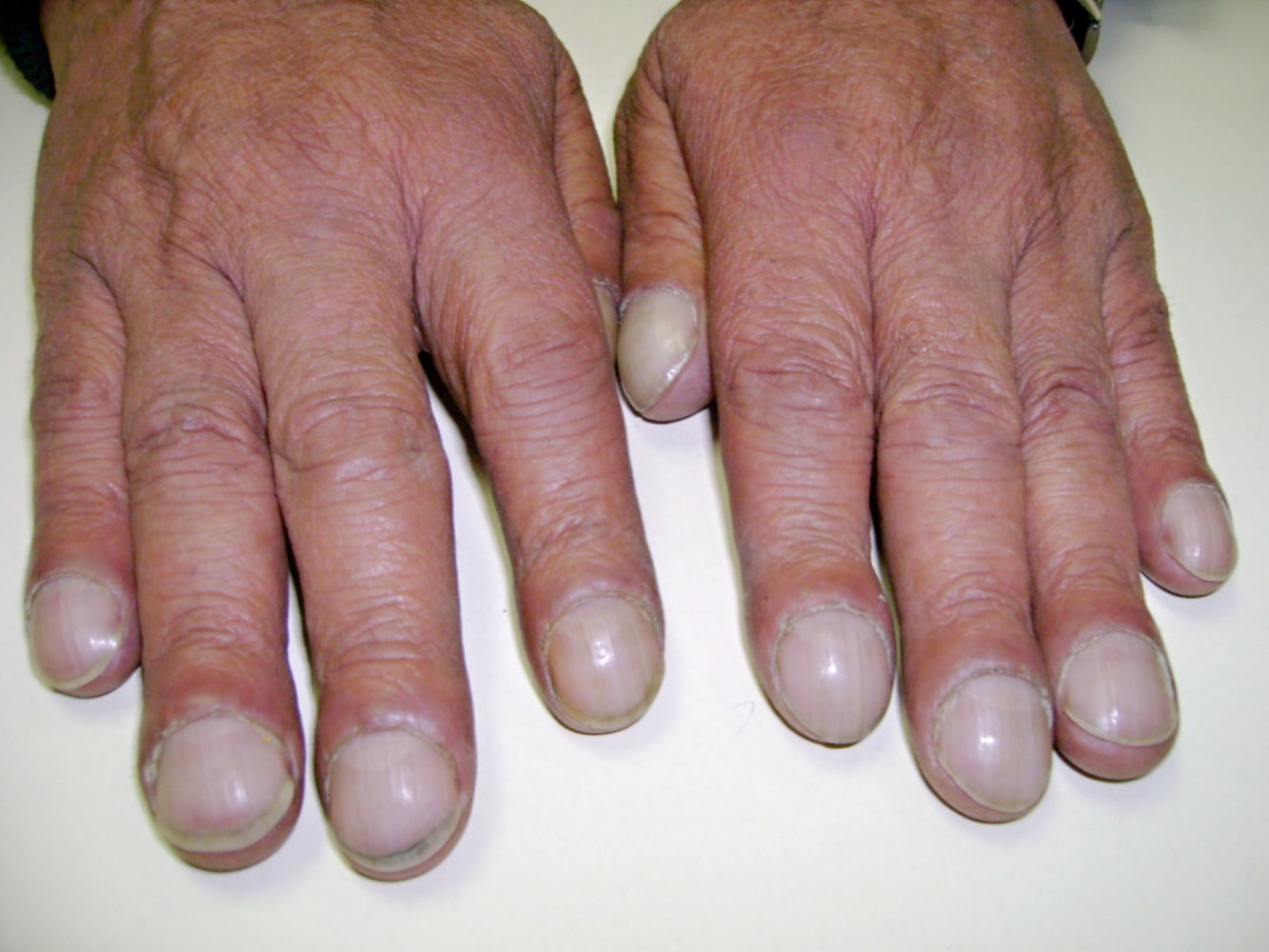
Diagnostics [3][10][57]
-
Imaging studies
-
Echocardiography is used for:
- Assessment of the underlying ACHD
- Evaluation of shunt direction
- Estimation of RV and pulmonary arterial pressures
-
Cardiac catheterization is used for: [10]
- Evaluation of shunt extension
- Measuring the pressure in the heart and pulmonary circulation
- Assessment for other conditions that may contribute to the right-to-left shunt
-
Echocardiography is used for:
- ECG: to monitor for the development of arrhythmias, e.g., atrial fibrillation
-
Laboratory studies
- CBC: ↑ Hb, ↑ Hct, and thrombocytopenia due to secondary erythrocytosis from chronic hypoxemia
- BMP: ↑ creatinine, ↑ BUN
- Iron studies: iron deficiency due to secondary erythrocytosis
Management [3][10][57]
Management should be guided by a specialist in pulmonary hypertension and either a pediatric cardiologist or a cardiologist specializing in CHD in adults depending on patient age.
- Medical management
- Counsel on exhibiting caution with exercise and request cardiopulmonary exercise testing.[57]
- Inform patients that Eisenmenger syndrome increases the rate of maternal mortality (30–50%) and fetal loss or morbidity (∼ 30%): [57]
- Offer contraception counseling as required.
- Offer elective termination of pregnancy as required.
- Assess for and manage complications, e.g., arrhythmias, kidney disease, and iron deficiency.
- Manage pulmonary hypertension: bosentan with the possible addition of PDE-5 inhibitors (e.g., sildenafil, tadalafil) for symptomatic management
- Heart and/or lung transplant with concomitant correction of the underlying ACHD (rare)
ACHD and pregnancy [58][59]
- The hemodynamic demands of pregnancy increase the risk of cardiovascular morbidity and mortality in individuals with ACHD.
- Refer patients with ACHD to a cardiologist for pre-pregnancy counseling and risk assessment; and follow-up during pregnancy and postpartum.
- The management of ACHD in pregnancy should involve a multidisciplinary care team (e.g., cardiology, maternal-fetal medicine, and anesthesiology).
Contraception [58][60]
The CDC offers an app to guide contraceptive method selection based on the US Medical Eligibility Criteria for Contraceptive Use and the US Selected Practice Recommendations for Contraceptive Use. See “Tips and Links.”
- Offer contraception counseling and tailor contraceptive choice, in consultation with cardiology, based on the following: [58]
- Patient preference
- Contraception contraindications
- Type and severity of the cardiac condition
- Risks and benefits of the contraceptive agent on the cardiac condition
- Choice of reversible contraception: [58][60][61]
- Progestin-only contraception is generally preferred for patients with valvular heart disease.
- IUDs are recommended for patients with high-risk cardiac conditions.
Contraceptives that contain estrogen are contraindicated for patients at high risk of thromboembolism. [10][62]
Planning pregnancy [10][58][61]
- Offer initial counseling on associated risks, e.g.:
- Increased risk for prematurity and CHD in the fetus
- Long-term worsening of the patient's cardiovascular condition
- Teratogenicity related to pharmacological treatment
- Counsel individuals at high risk to avoid pregnancy and consider reproductive alternatives (e.g., surrogacy, adoption). [58][59]
- Offer genetic counseling. [10][58]
- Refer to cardiology for appropriate evaluation and optimization of management of ACHD before pregnancy, including:
- Maternal risk assessment
- Cardiac testing (e.g., TTE, exercise stress testing)
- Consideration for surgical correction of ACHD before pregnancy
- Adjusting medications to avoid teratogenicity: See "Pregnancy restrictions on heart failure medications.”
During pregnancy [10][58][59]
Refer all patients to an obstetrician specializing in complex pregnancies and a cardiologist specializing in ACHDs for multidisciplinary care.
- Offer elective pregnancy termination when appropriate to patients with high-risk conditions, e.g.,: [10][58][59]
- Pulmonary arterial hypertension of any cause, including Eisenmenger syndrome
- Severe systemic ventricular dysfunction (i.e., LVEF < 30%, NYHA III–IV)
- Moderate or severe complex disease (e.g., NYHA IV symptoms, severe hypoxemia, refractory arrhythmias)
- Obtain antenatal imaging. [58][59]
- TTE
- Fetal echocardiography at 18–22 weeks' gestation
- Serial imaging to evaluate for fetal growth restriction
- Review pregnancy restrictions on heart failure medications and switch to safer agents if feasible.
- See “Management of high-risk pregnancies” for considerations related to delivery.
Pregnancy termination should be considered in patients who have major fetal and/or maternal risk factors. [63]