Adaptive (acquired) immunity is a part of the immune system that provides an antigen-specific response following exposure to a microbial pathogen or foreign substance (e.g., antigen). The adaptive immune system primarily involves B cells, T cells, and circulating antibodies, all of which mount a targeted immune response to a particular antigen/invading pathogen. An important component of adaptive immunity is immunologic memory, a mechanism by which the immune system forms memory B cells and memory T cells. These cells are able to trigger a more rapid and extensive response following subsequent antigen exposure. Adaptive immunity can be conferred via vaccination, which induces immunity through selective exposure to antigens that have been rendered innocuous. Autoimmunity is a disorder of the adaptive immune system and is characterized by immune responses to the body's own tissue. Immunodeficiency conditions, in which a compromised immune system leaves the body highly susceptible to infections, can be either congenital (see “Congenital immunodeficiency disorders” for more information) or acquired (e.g., HIV infection, iatrogenic immunosuppression).

Overview [1][2][3]
- A major component of the adaptive immune response
-
Essential for cell-mediated immunity
- Directly involves cytotoxic T cells and other phagocytes, but not B cells or antibodies
- Important in the recognition and destruction of intracellular pathogens (e.g., viruses, intracellular bacteria)
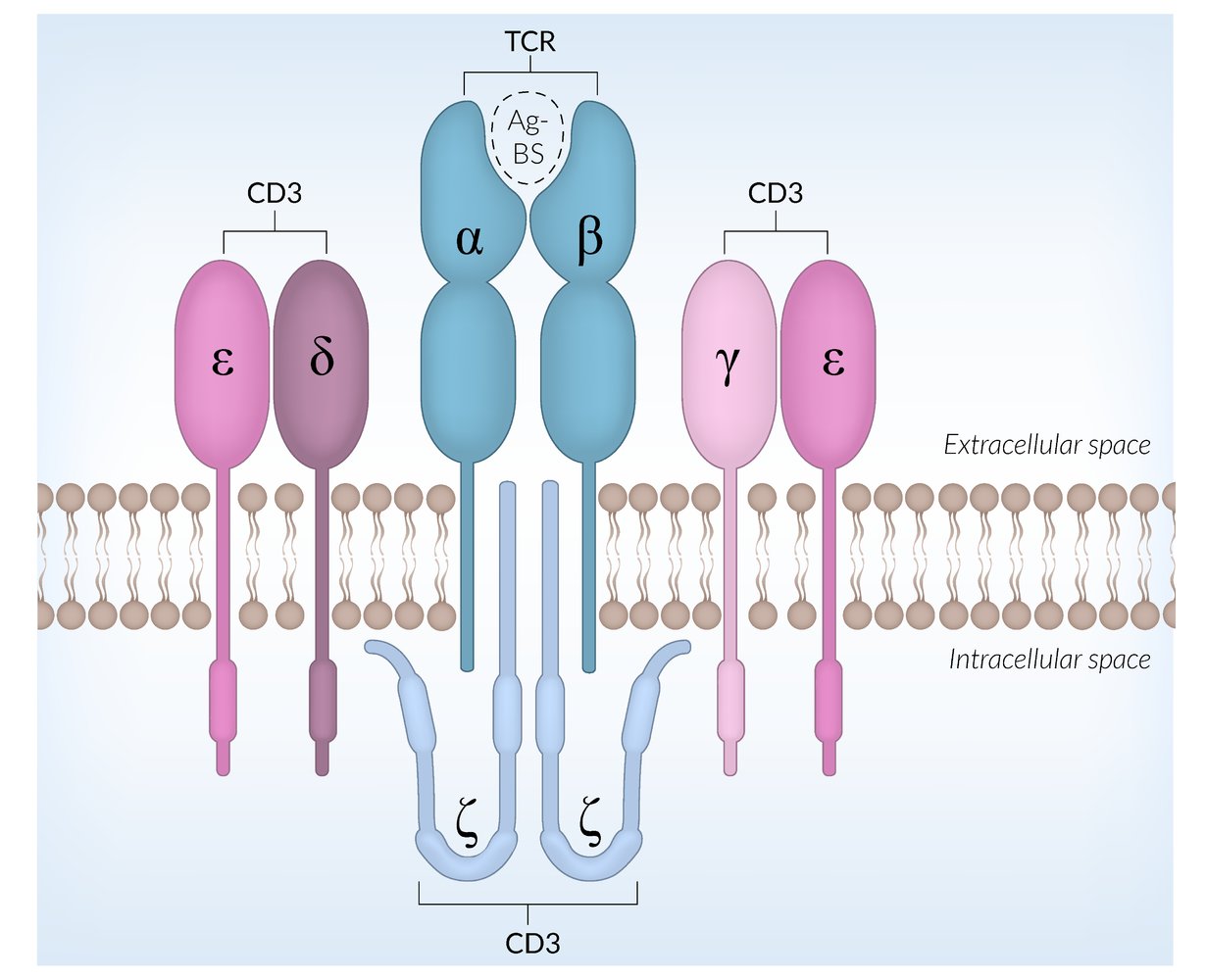
- All mature T cells express specific surface proteins that distinguish them from other lymphocytes and allow them to recognize antigens presented by MHC molecules of antigen-presenting cells.
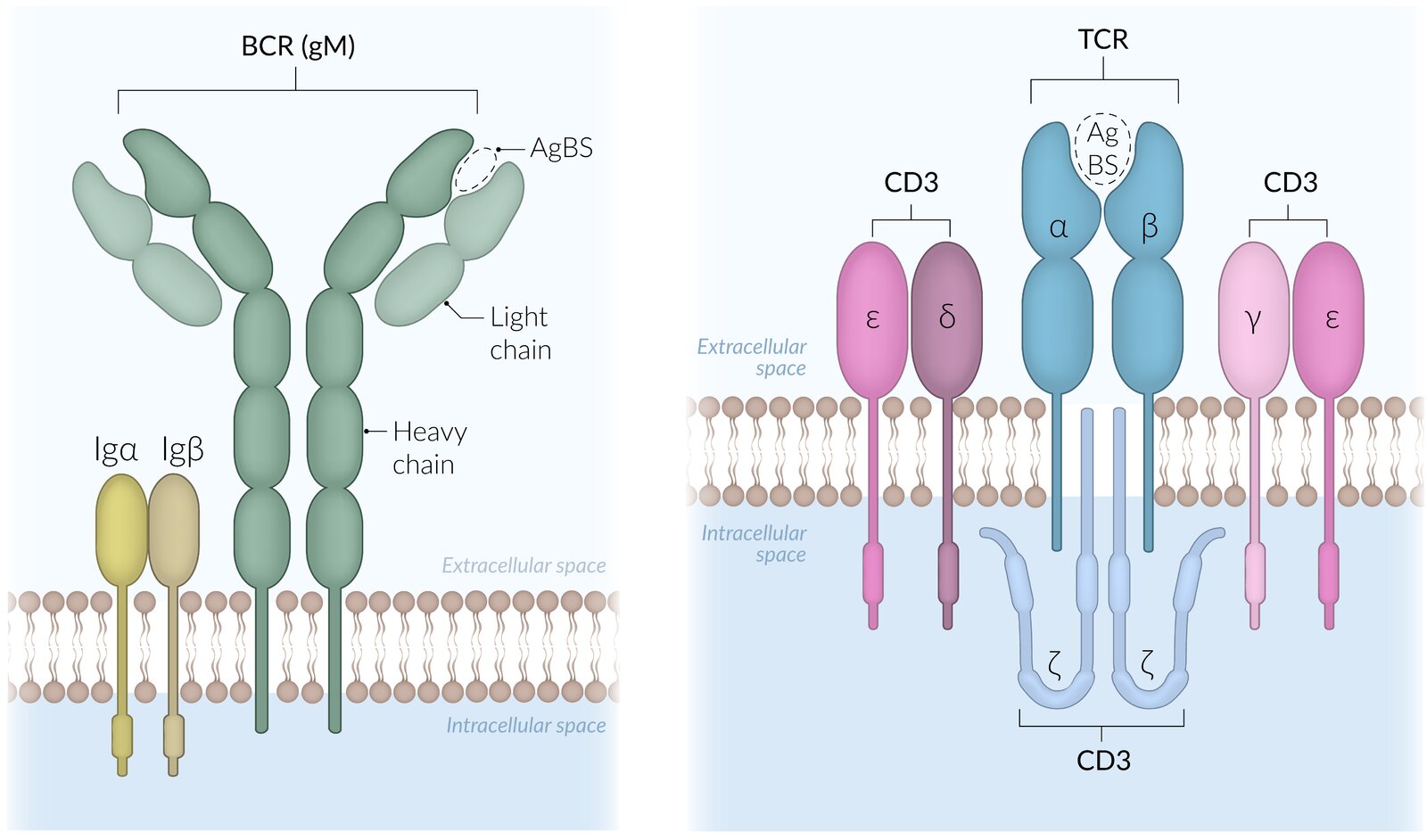
T-cell receptors (TCRs)
- Complex of proteins of the immunoglobulin superfamily
-
Each T cell expresses a TCR variant that binds to one specific antigen.
- Binding of a TCR to its specific antigen initiates T cell activation (see “T-activation” below).
- The antigen fragment has to be presented by a MHC molecule of an antigen-presenting cell in order to be recognized by the TCR.
- Important for positive and negative selection during T-cell development
The cells of the acquired immune system (B cells, T cells) are activated upon antigen recognition.
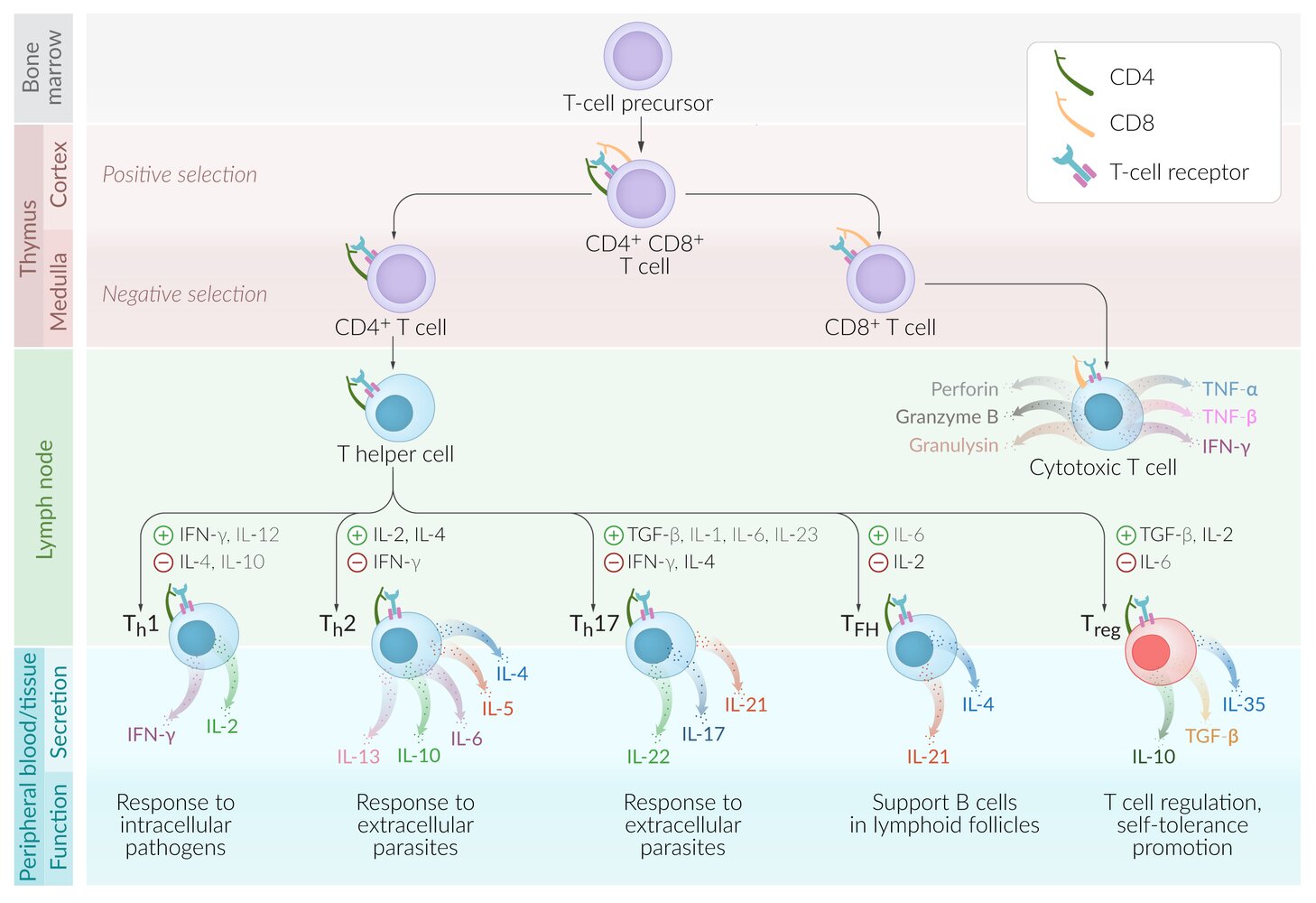
T-cell development [4]
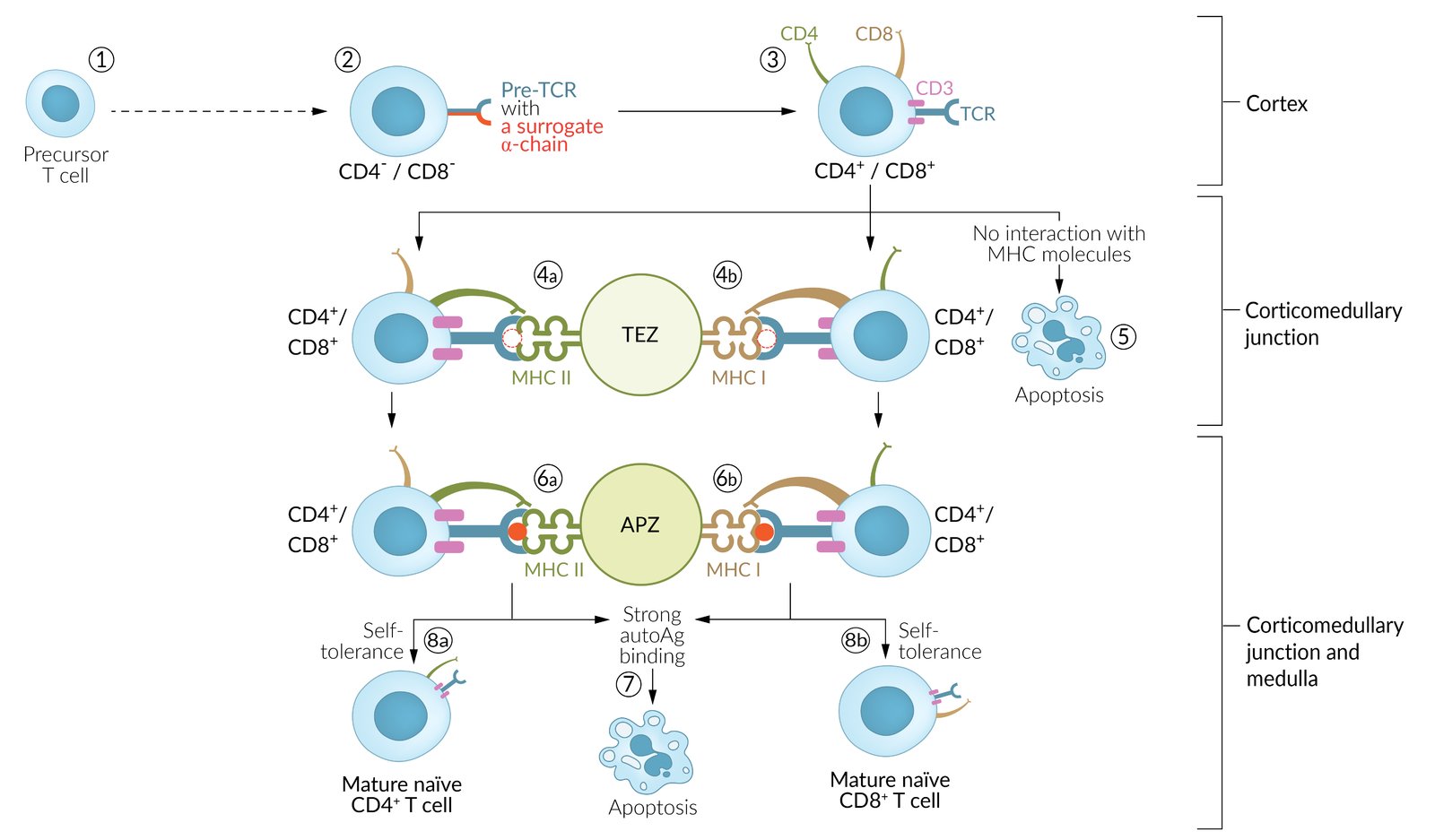
T cells originate from lymphoid progenitor cells in the bone marrow and mature in the thymus.
-
Positive selection of T cells: ensures that the thymus produces functionalT cells
- Location: thymic cortex
- Thymic cortical cells express MHC class I and MHC class IIantigens.
-
Tests if T-cell receptors can bind to MHC appropriately (not too strongly or too weakly)
- T cells (CD4+/CD8+, double-positive thymocytes) receive survival signal.
- Dysfunctional T cells then undergo apoptosis.
-
Negative selection of T cells: ensures that the thymus does not produce self-reactingT cells
- Location: thymic medulla
-
Tests if T cells bind to tissue-restricted self-antigens presented on MHC by thymic medullary cells
- T cells that do not bind receive survival signal.
- T cells that bind self-antigens undergo apoptosis, except for a few that become regulatory T cells.
-
Self-antigen presentation is mediated by the autoimmune regulator protein(AIRE protein), dysfunction of which can lead to:
- Adrenal insufficiency
- Chronic mucocutaneous candidiasis
- Hypoparathyroidism
-
In addition, T cells bind with their cluster of differentiation (CD).
-
The type of CD that the T cell has a higher affinity for is kept, while the other is downregulated to either (CD4+/CD8-) or (CD4-/CD8+).
- CD4 binds to MHC II.
- CD8 binds to MHC I.
-
The type of CD that the T cell has a higher affinity for is kept, while the other is downregulated to either (CD4+/CD8-) or (CD4-/CD8+).
- Immunocompetent, but still naive T cells leave the thymus and migrate within and between peripheral tissues, blood vessels, and secondary lymphoid organs (e.g., lymph nodes, spleen, MALT).
Defective negative T-cell selection can cause autoimmune disorders (e.g., type 1 autoimmune polyendocrine syndrome).
“Life is ACHe without AIRE”: Autoimmune regulator protein (AIRE) dysfunction can lead to Adrenal insufficiency, chronic mucocutaneous Candidiasis, and Hypoparathyroidism.
T-cell activation
Overview
- Antigens are processed by antigen-presenting cells (i.e., macrophages, monocytes, B cells, Langerhans cells, and dendritic cells).
- Dendritic cells travel through different tissues, phagocytize and process antigens; and then migrate through afferent lymphatic vessels to a lymph node to present these antigens via MHC I and II.
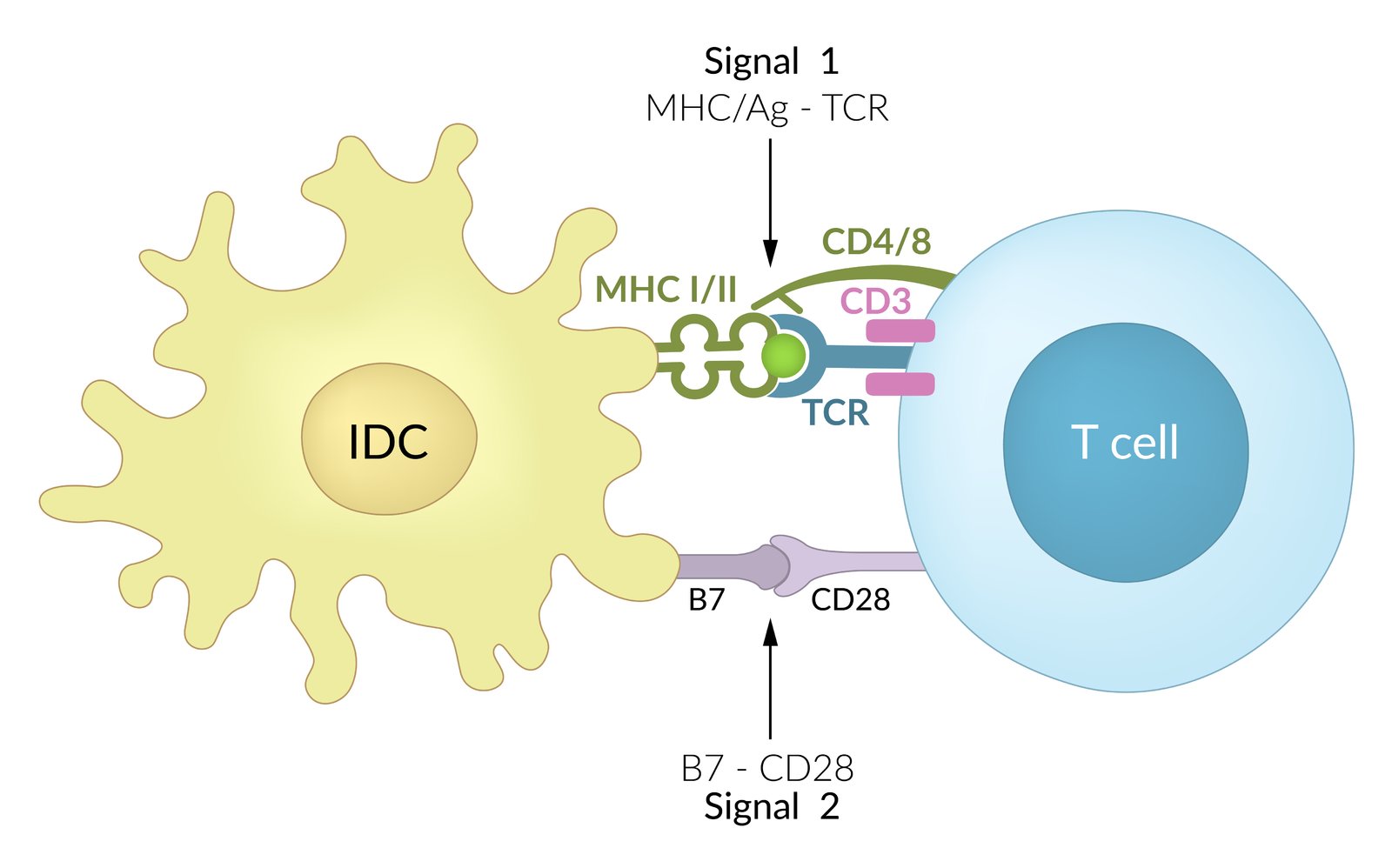
- T cell activation (“priming”); mainly occurs in secondary lymphoid organs, such as lymph nodes.
- Activation requires an initial signal, followed by a second, costimulatory signal.
Mechanism
-
Antigen presentation
- Performed by antigen-presenting cells
- Exogenous antigens are presented via MHC II to TCR/CD4.
- Endogenous antigens are presented via MHC I to TCR/CD8 (cross-presentation of antigens).
-
Costimulatory signal: mediates survival and proliferation of T cells
- B7 protein (CD80 or CD86) on the dendritic cell: interacts with CD28 on the naive T cell.
-
Antigen presentation without this co-stimulatory signal will lead to T-cellanergy:
- Important self-tolerance mechanism
- The cell will not be activated even though it is exposed to its antigen
- Superantigens (e.g., toxic shock syndrome toxin 1, enterotoxin B) link MHC II antigen-presenting cells and T-cell receptors on T cells and lead to activation of T cells without a costimulatory signal.
-
Effect
- T-helper cell: activation of NFAT (nuclear factor of activated T cells) → cytokine production → activation of other cells, e.g., B cells, macrophages, and cytotoxic T cells
- Cytotoxic T cell: recognizes and destroys cells that possess antigens (signals malignancy or viral infection)
T cell effects
-
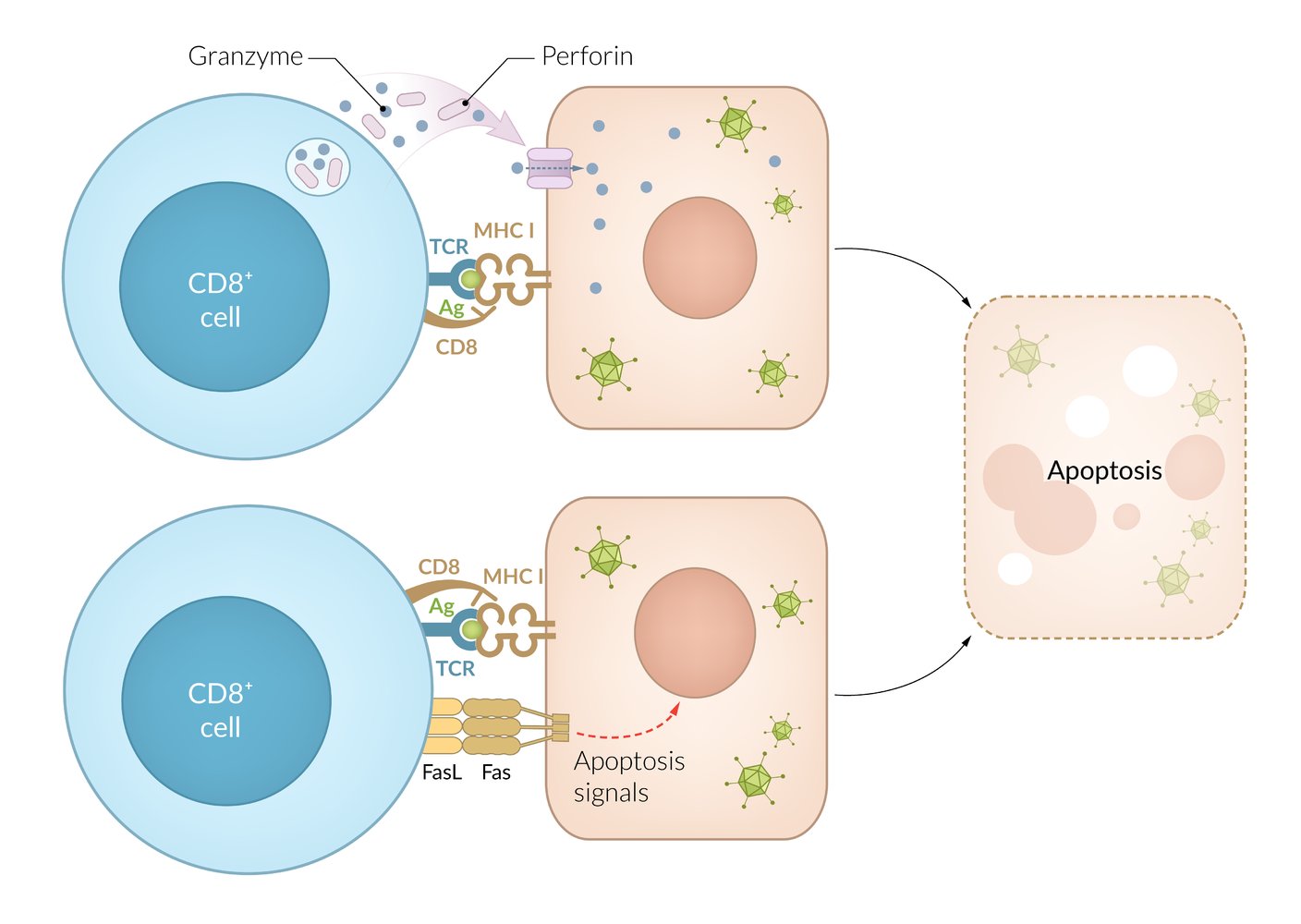
T cells (CD8+): direct cell lysis or induction of apoptosis via perforin and proteases
- Activated via antigen presentation by MHC class I receptors
- Induce apoptosis of virus-infected or malignant cells using mechanisms as those used by NK cells: release of granules that contain perforin, granzyme B, granulysin
- Release cytokines (including IFN‑γ, TNF, and lymphotoxin-α) → macrophage activation
- Clinical relevance: involved in organ rejection (acute and chronic), induce apoptosis of donor graft cells
-
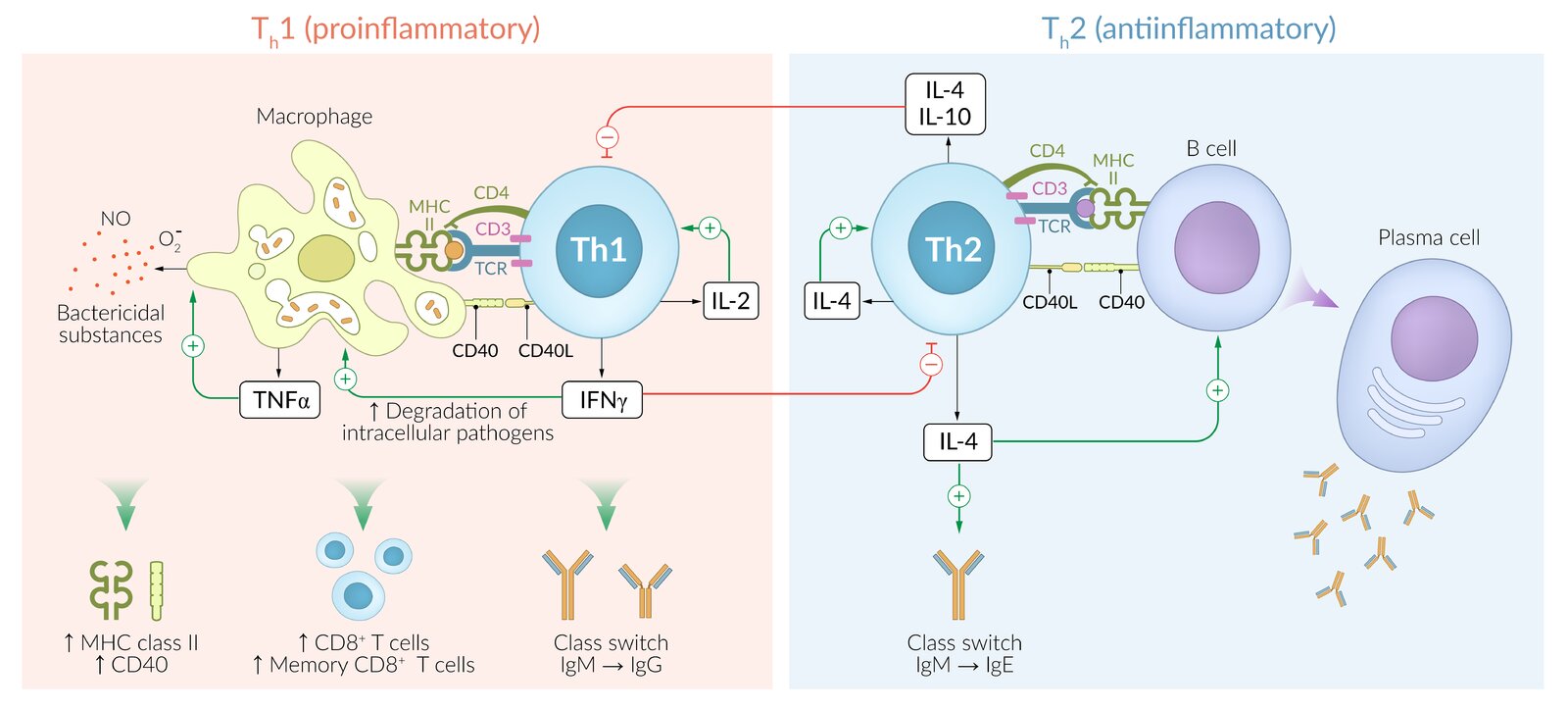
Th1 cell (CD4+): cell‑mediated response
- Activated via antigen presentation by MHC class II receptors
-
Immune response to intracellular pathogens (viruses, intracellular bacteria)
- Release cytokines (including IFN‑γ, IL‑2, and TNF) → stimulation of macrophages (positive feedback) and CD8+ cytotoxic T cells; ; effect is amplified by binding of T-cell CD40L to CD40 receptors on macrophages
- IFN‑γ, IL-2, and TNF‑α induce granuloma formation against foreign bodies that cannot be eliminated by the immune cells.
- Clinical significance:
- Tuberculosis in HIV infection
- Delayed cell-mediated type IV hypersensitivity reactions (see the corresponding section in ”Hypersensitivity reactions”)
-
Th2 cell (CD4+): cell‑mediated response
- Activated via antigen presentation by MHC class II receptors
-
Immune response to extracellular pathogens (bacteria, parasites)
- Release cytokines (including IL-4, IL-5, IL-13) which stimulate eosinophils, basophils, and mast cells
T cell subtypes
- T cells are largely divided into cytotoxic T cells (CD8+), T helper cells (CD4+), and regulatory T cells.
- Other subtypes include memory T cells and natural killer T cells.
| Overview of T cell subtypes | |||||
|---|---|---|---|---|---|
| Cell type | Important surface markers | Function | Stimulate/activate | Clinical significance | |
| Cytotoxic T cells (killer T cells) |
|
|
|
|
|
| T-helper cells (Th cells) | Th1 cells |
|
|
|
|
| Th2 cells |
|
|
|
||
| Th17 cells |
|
|
|
||
| T follicular helper cells (TFH cells) |
|
|
|
||
| Regulatory T cells (Treg, suppressor T cells) |
|
|
|
|
|
Surface markers
Surface protein expression determines the specific function of T cell subtypes.
-
General T cell markers
- All T cells carry membrane-bound marker proteins that distinguish them from other lymphocytes.
- These general T-cell markers are CD3, CD28, and the T-cell receptor (TCR).
-
Specific markers of T cell subtypes
-
T cells are largely divided into CD8+ T cells (cytotoxic T cells) and CD4+ T cells (T helper cell subpopulations).
- Besides CD4, T helper cells express CD40L, CXCR4, and CCR5.
- See also “CD4/CD8 ratio.”
- Subtypes within the group of CD4+ T cells may be identified by the cytokines they secrete and/or by their specific surface markers (see table below; the list is not exhaustive).
-
T cells are largely divided into CD8+ T cells (cytotoxic T cells) and CD4+ T cells (T helper cell subpopulations).
| Differentiation of T helper cell subtypes | ||||
|---|---|---|---|---|
| Cell type | Surface marker | Stimulated by | Cytokines produced | Inhibited by |
| Th1 cell |
|
|
|
|
| Th2 cell |
|
|
|
|
| Th17 cell |
|
|
|
|
| TFH cell |
|
|
|
|
| Treg cell |
|
|
|
|
CD8 proteins on the surface of cytotoxic T cells interact with MHC I receptors, while CD4 proteins on the surface of T-helper cells interact with MHC II receptors.
Rule of 8: MHC I x CD 8 = 8. MHC II x CD 4 = 8.
Overview [1][2][3][5]
- Major component of the adaptive immune system: The humoral immune response of the adaptive immune system mainly consists of B cells and antibodies.
B-cell development
- Originate and mature in the bone marrow
- Naive B cell: a mature B cell that has not come in contact with antigens yet
-
Express numerous surface proteins
- CD19, CD20, CD21 (used by EBV), and CD40
- MHC II
- B7
- B cell receptor with immunoglobulin component (binds antigens)
- Mature B cells circulate between the blood and secondary lymphatic organs (e.g., lymph nodes, spleen, MALT).
- After activation, B cells differentiate into plasma cells that produce and secrete antibodies (see “Immunoglobulins” below).
“Mr. Epstein, you have to be 21 to Be in this Barr!”: The Epstein-Barr virus uses the CD21 receptor to invade B cells.
B-cell receptors (BCRs)
- Type I transmembrane proteins on the B cell surface that are composed of immunoglobulins and signal-transmitting subunits
- Necessary for B cell activation and maturation
- The immunoglobulin parts of mature BCRs are highly specific to certain antigens.
- Naive BCR: a BCR that has not interacted with an antigen yet
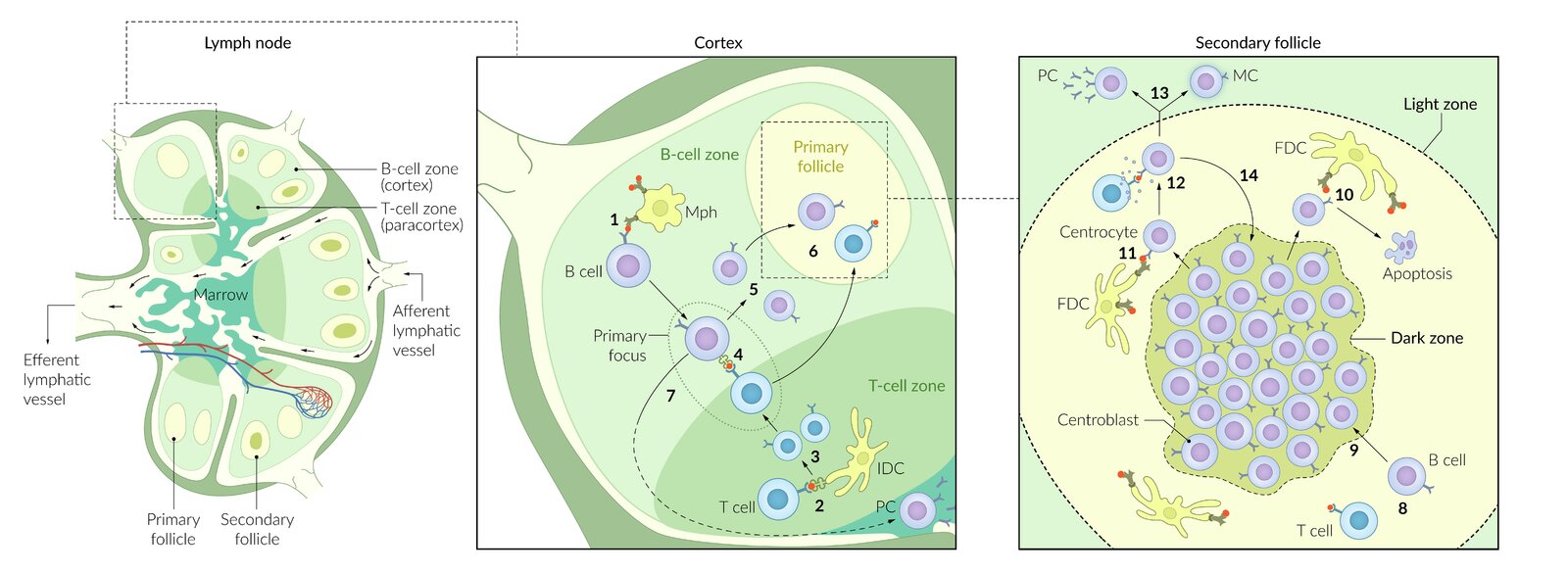
B cell activation
B cell activation and class switching require an initial signal, as well as a costimulatory signal. Activated B cells migrate to germinal centers of secondary lymphoid organs.
-
T cell-dependent activation of B cells
- Requires activation of CD4+ T-helper cells, which, in turn, requires prior antigen presentation via MHC II by the B cell
- Initiated as a response to protein or peptide antigens (thymus-dependent antigens), e.g.:
- Pneumococcal conjugate vaccines
- Diphtheria toxin-like protein with conjugated polysaccharides
- B lymphocytes recognize antigens via their B-cell receptors (membrane‑bound immunoglobulins, IgD or IgM) → B cell receptor-mediated endocytosis of the BCR/antigen complex → breakdown of antigen into small fragments by lysosomal proteases → presentation of antigen fragment via MHC class II receptors on B cell surface to Th cells ; plus costimulation of B cell CD40 receptor by Th cell CD40L → T cell‑dependent activation of B cells (plasma cells) → immunoglobulin production
-
T cell-independent activation of B cells
- Initiated as an immediate response to nonprotein antigens (thymus-independentantigens; e.g., gram-negativelipopolysaccharide)
- Leads to production of IgM antibodies
- No prior T-cell activation since MHC are unable to present nonprotein antigens to T cells
- Thymus independent antigens are considered to be only weakly immunogenic, which is why vaccines containing nonprotein antigens need adjuvants (like the PPSV23 Streptococcus pneumoniae vaccine, containing a capsular polysaccharide subunit) and boosters.


Affinity maturation
- Definition: : A process in which B cells interact with Th cells within the germinal center; of secondary lymphoid tissue in order to secrete immunoglobulins with higher affinity for specific antigens.
-
Mechanisms
- Somatic hypermutation: point mutations that create random alterations in the variable region of the antibody gene
- Clonal selection: B cells that possess antibodies with higher affinity for the antigen have a survival advantage through a positive selection that allows them to proliferate and predominate within the follicle.
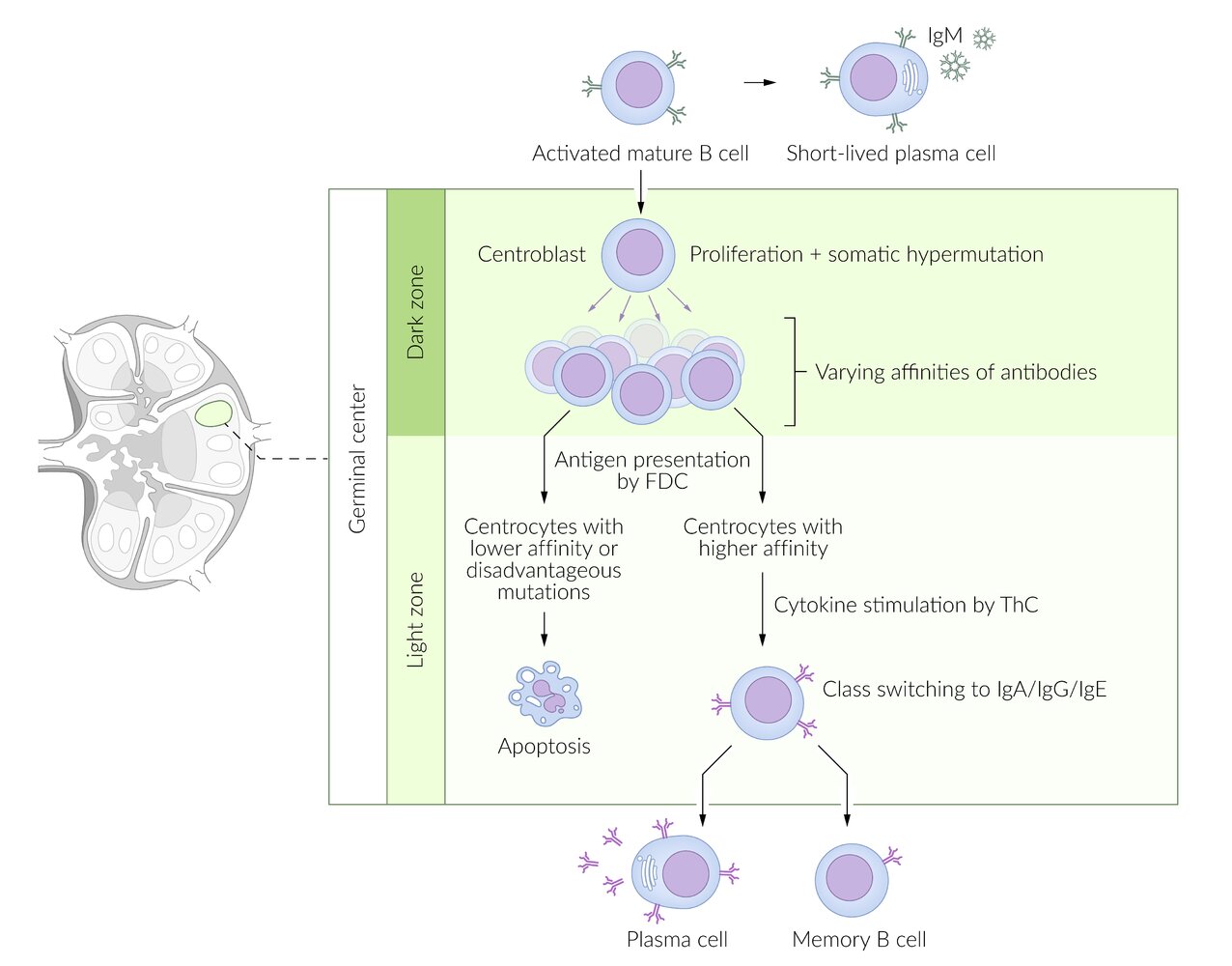
Isotype switching (class switching)
Within the germinal centers of lymph nodes, activated B cells change the antibody isotype in response to specific cytokines that are released by Th cells. IgM, the primary antibody on B cells before getting activated, is switched to IgA, IgE, or IgG. IgM is also secreted by plasma cells (stimulated by IL-6).
-
B cellclass switching occurs via two signaling mechanisms:
- First signal = activation: Antigen bound to MHC II molecule binds to T-cell receptor on the surface of T-helper cells.
-
Second signal = costimulation: CD40membrane receptor on the B cell binds to CD40 ligand (CD40L) on the surface of CD4+ T cell (CD40L/CD40) → cytokinerelease →gene rearrangement, resulting in class switching
- IL-4, IL-13: stimulate class switching to IgE.
- IL-5, TGF-β: stimulate class switching to IgA.
- IFN-γ, IL-4, IL-21: stimulate class switching to IgG.
- The resulting antibody has the same affinity for the antigen but a different function.
- Isotype switching is irreversible.
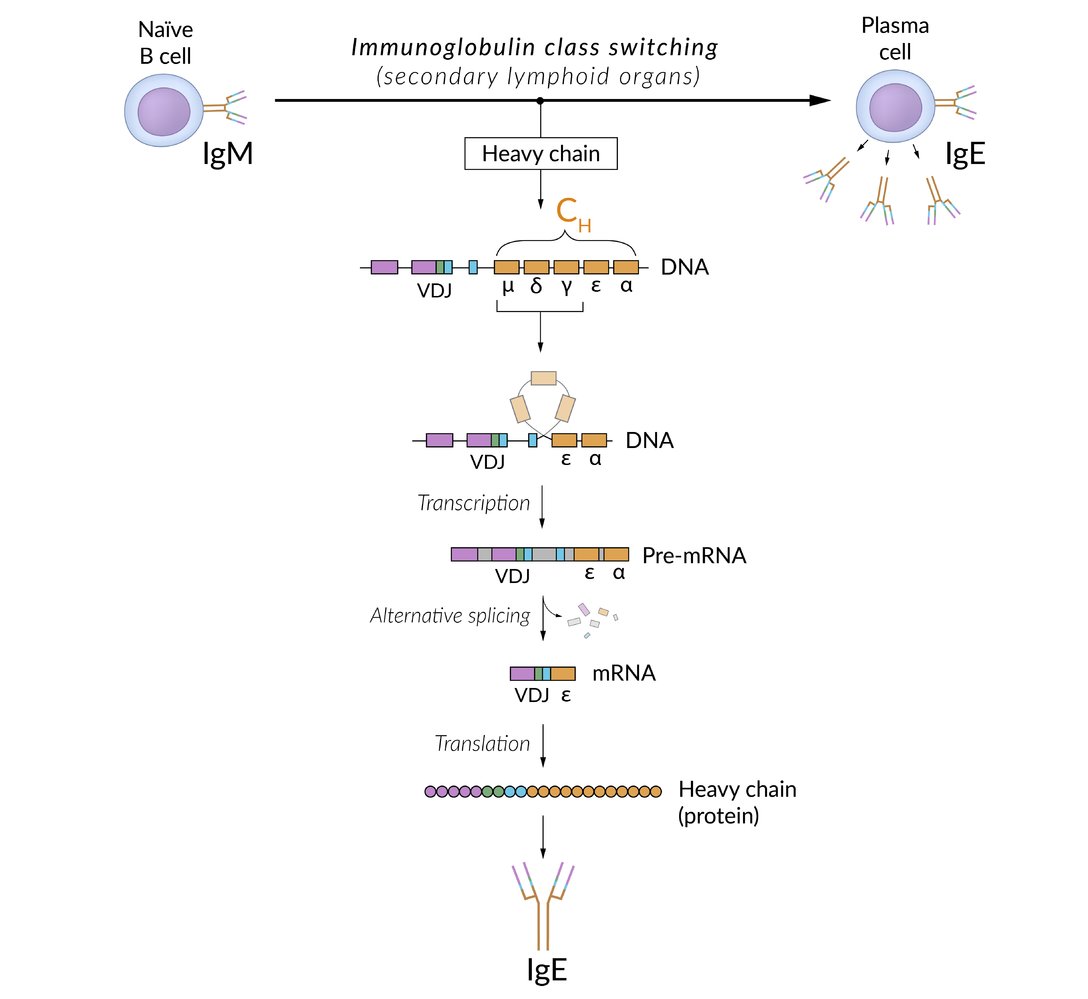
Class switching occurs with AGE: IgA, IgG, IgE.
Overview
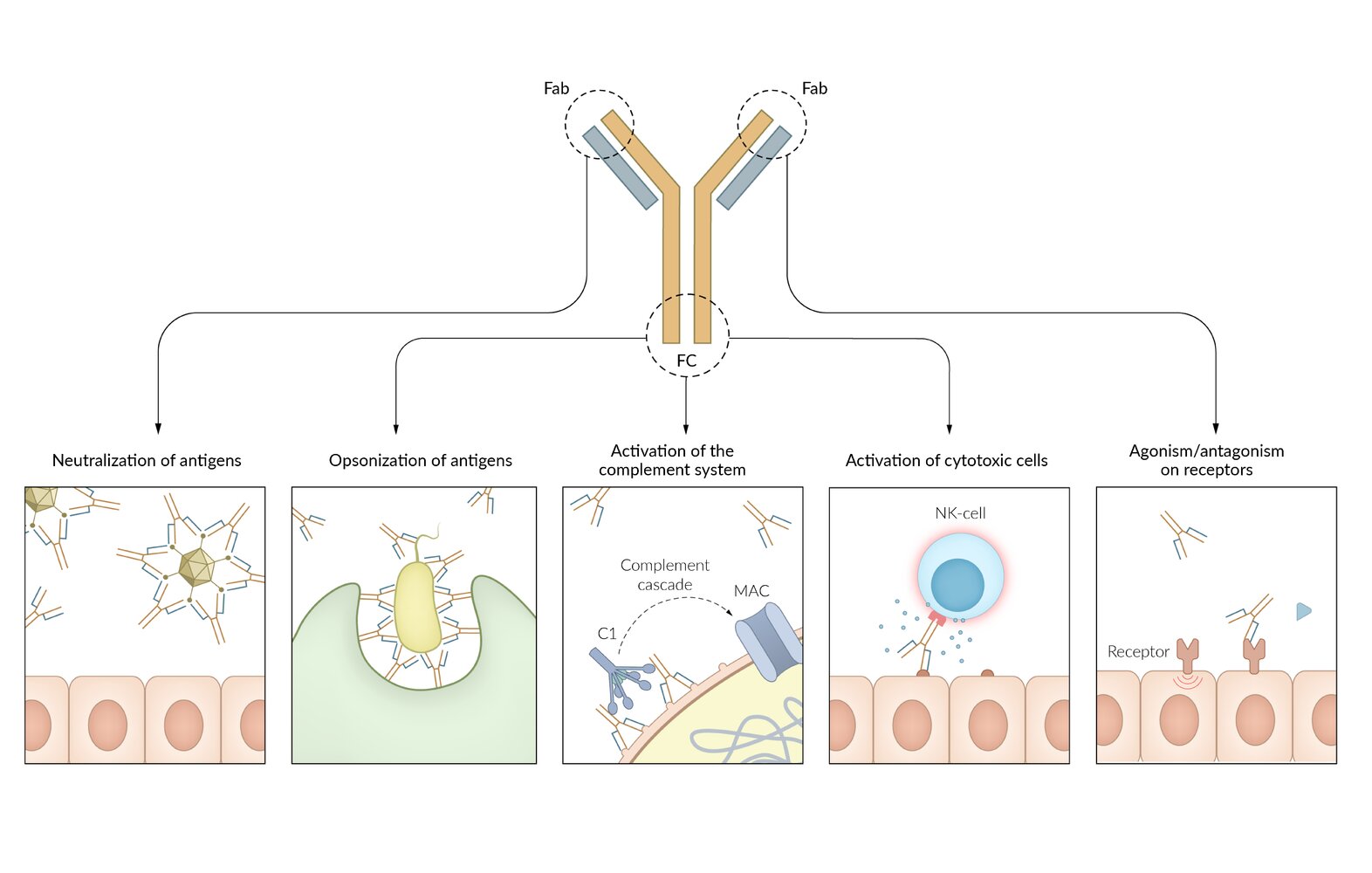
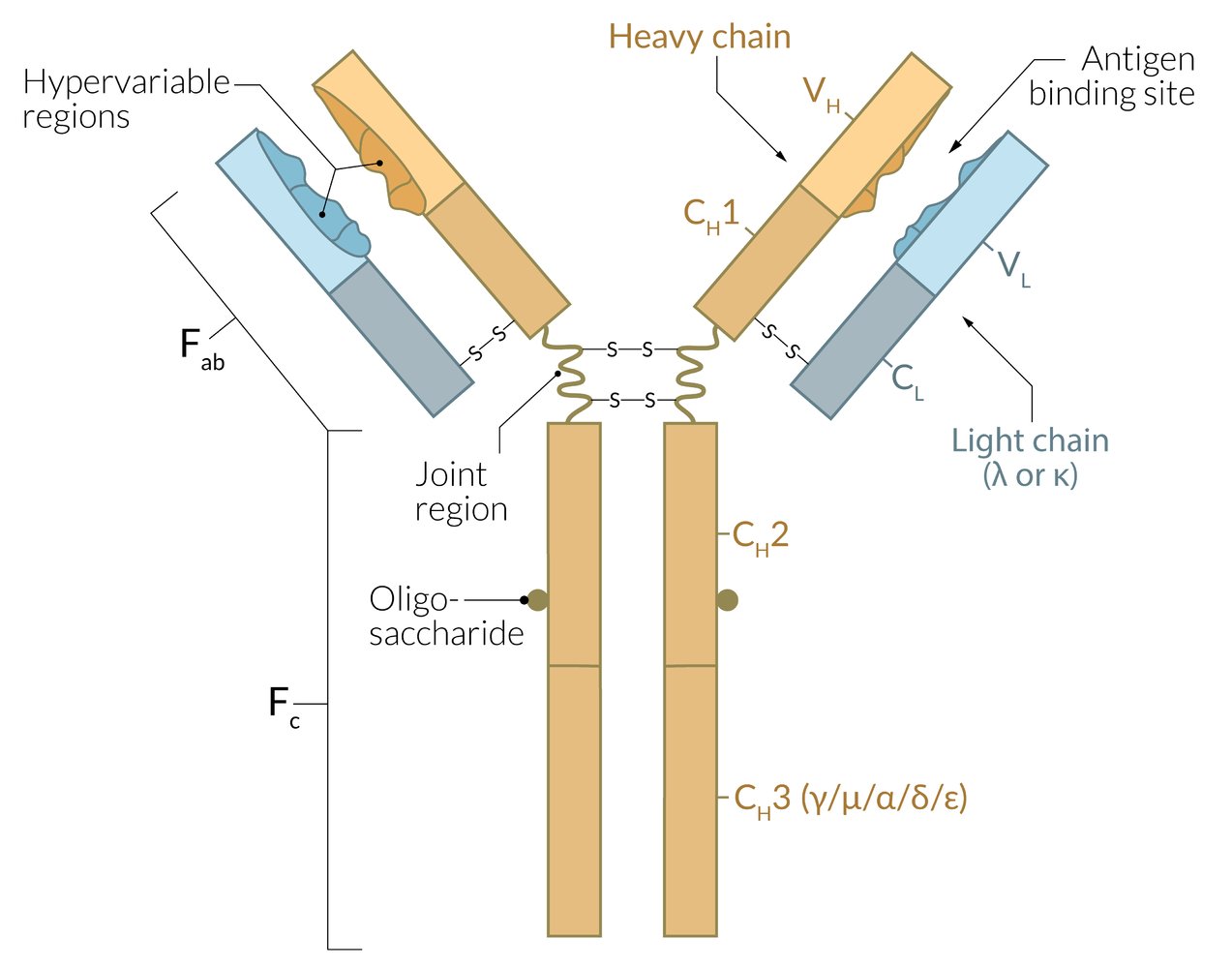
Immunoglobulins (antibodies) have two functional parts: the Fc region and the Fab region; . The two enzymes papain and pepsin can be used to identify the different functional parts. Every immunoglobulin can have monomeric structure. In context of immunoglobulins, the term affinity refers to individual interaction of antibody and antigen, whereas avidity characterizes the accumulated binding strength of all antigen-binding sites combined.
-
Fc region
- Contains the constant region
- Formed by heavy (H) chains
- Determines the antibody isotype (e.g., IgA, IgG, IgM)
- Binds complement (IgG, IgM)
- Binds various immunological cells, such as macrophages, to stimulate phagocytic or cytotoxic activity (opsonization)
- Contains the carboxyl terminal
- Has many carbohydrate side chains
-
Fab region
- Contains the variable/hypervariable region
- Formed by light (L) chains and heavy (H) chains
- Recognizes and binds to antigens via epitope
-
Determines the idiotype
- Binding site with specificity for one particular kind of antigen
- Every B cell expresses exactly one antigenic specificity
Fc → Complement, Constant, Carboxy terminal, Carbohydrate side chains
Fab → Antigen binding
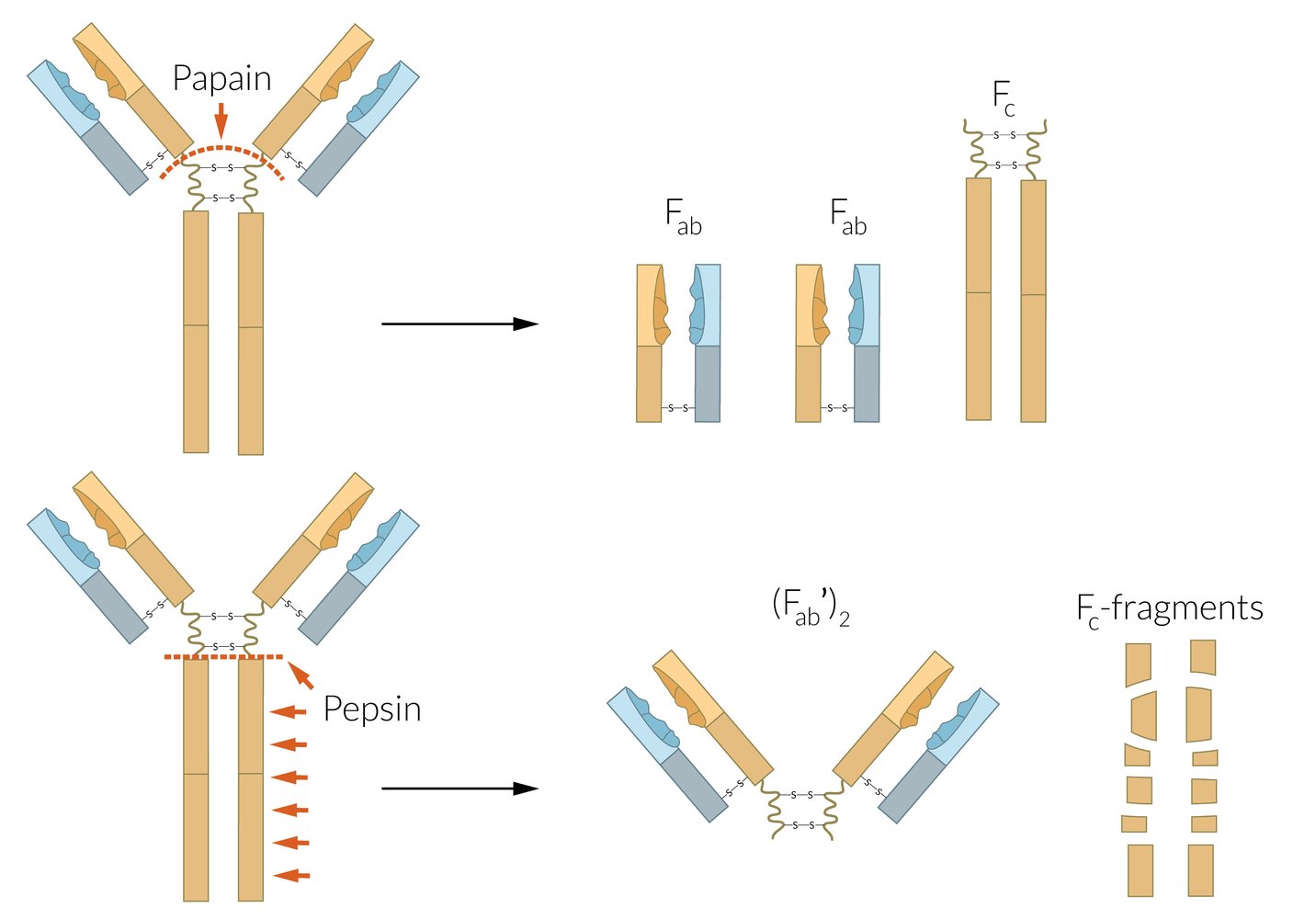
Immunoglobulin properties
Specificity
- Requires antigens
-
Occurs by somatic hypermutation and affinity maturation
- Alterations take place in the variable region.
- Normal response to antigenic stimulation: B lymphocytes with varying immunoglobulin alleles (i.e., polyclonal proliferation)
- Malignant lymphocyteproliferation: predominance of B lymphocytes with a single immunoglobulin variable domain (i.e., monoclonal proliferation)
-
Class switching (e.g., IgA, IgM, IgG)
- Alterations take place in the sequence of the heavy chain constant domain.
Diversity
- Does not require antigens
-
Recombination-activating genes (RAG): a set of genes that encode RAG proteins 1 and 2, which together form V(D)J recombinase, an enzyme required for V(D)J recombination in T cells and B cells
-
Random recombination of certain genes during B cell maturation in bone marrow
- Light chain: VJ genes
- Heavy chain: V(D)J genes
- Autosomal recessive RAG1 and RAG2 loss-of-function mutations are a rare cause of severe combined immunodeficiency.
-
Random recombination of certain genes during B cell maturation in bone marrow
- Terminal deoxynucleotidyl transferase (TdT) randomly adds nucleotides to the DNA.
- Recombination of light chains with heavy chains occurs randomly
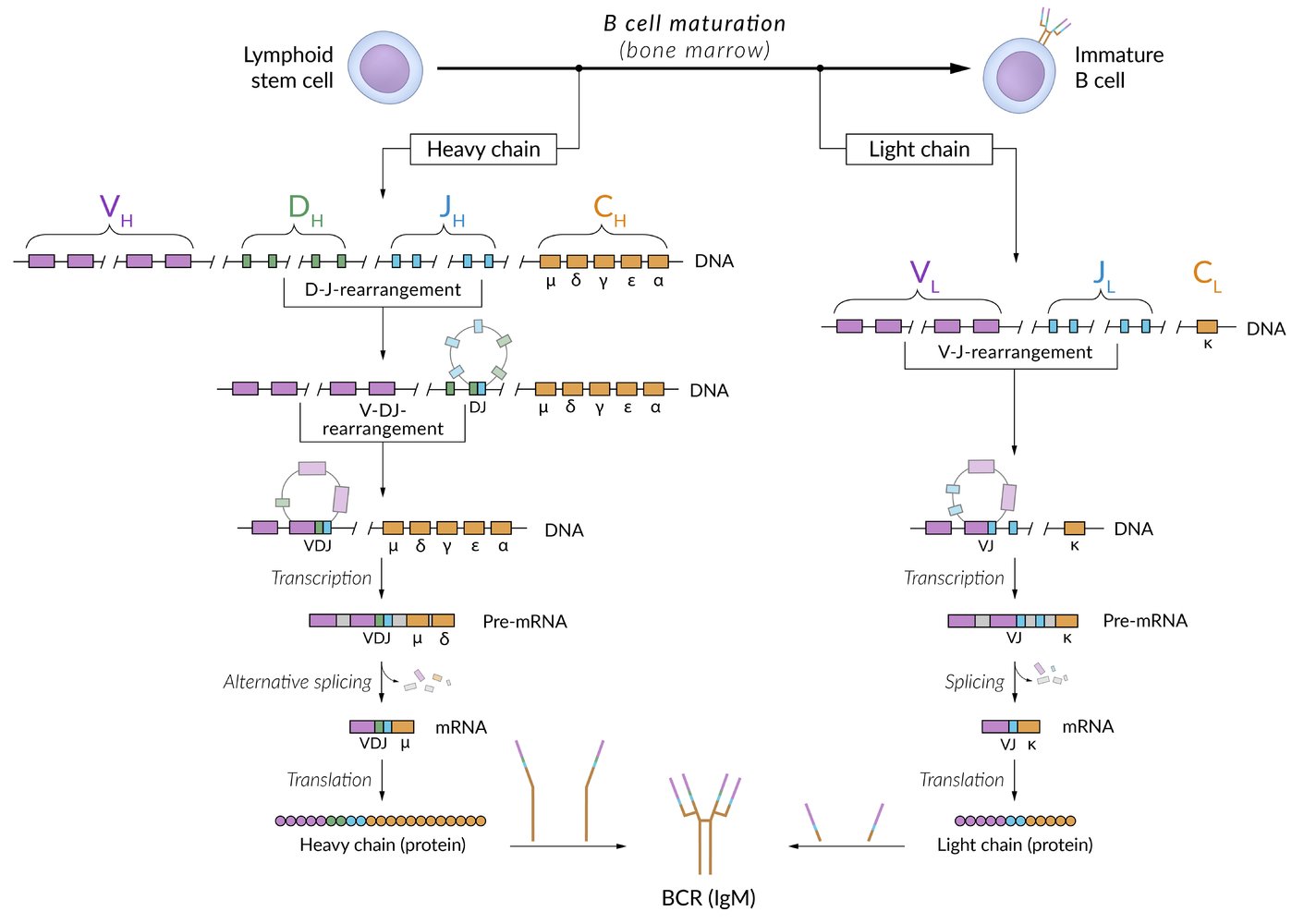
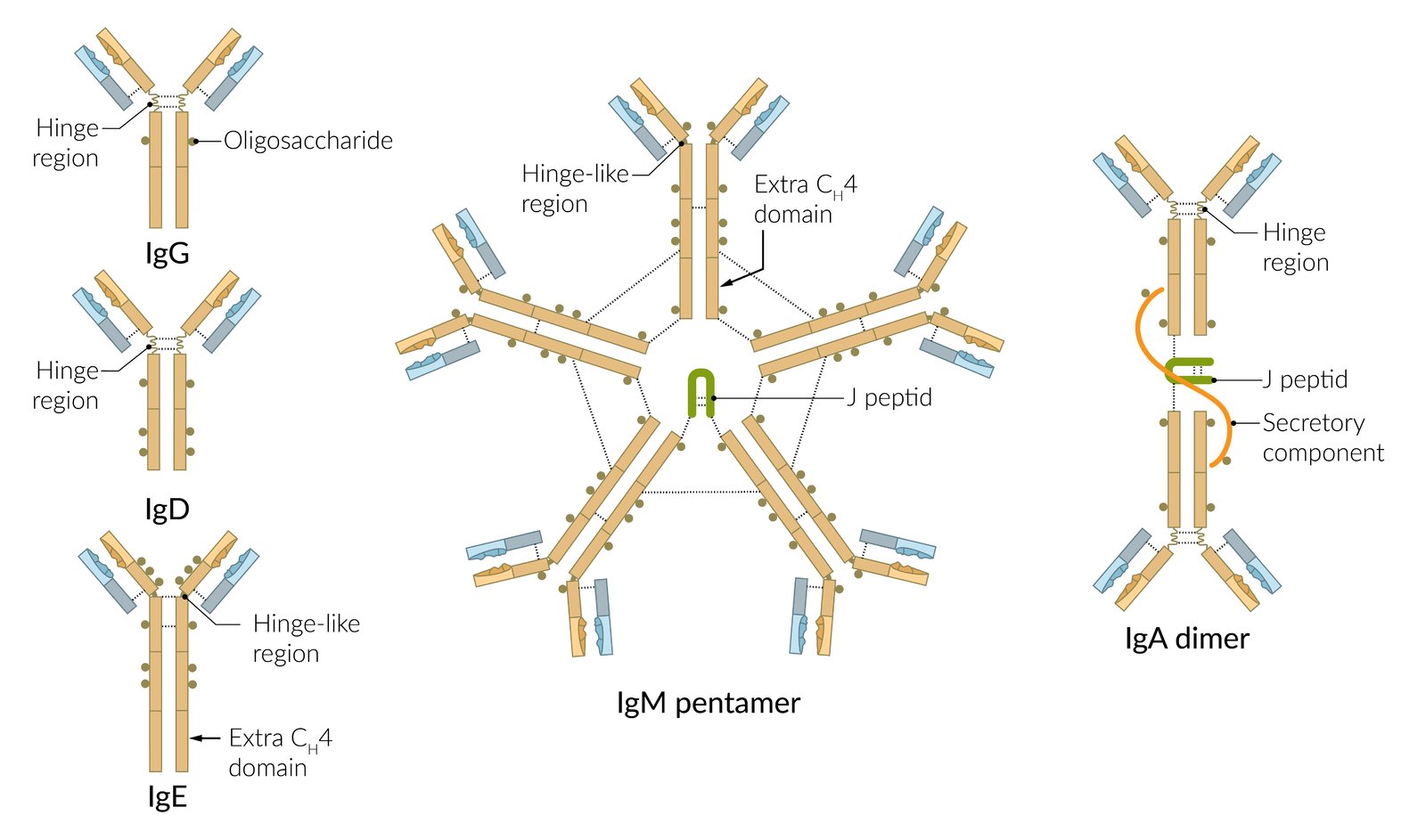
Immunoglobulin types
| Overview of immunoglobulins | |||
|---|---|---|---|
| Type | Structure | Characteristics | Examples and clinical relevance |
| IgM |
|
|
|
| IgG |
|
|
|
| IgA |
|
|
|
| IgE |
|
|
|
| IgD |
|
|
|
To memorize the timing of IgM formation, think of IgM as forming iMmediately!
To remember that IgG can cross the placenta and conveys transient passive immunity, think of “IgG Grants immunity to the Growing fetus”
IgA is an Intra-gut Antibody (mainly found in the gastrointestinal mucosa).
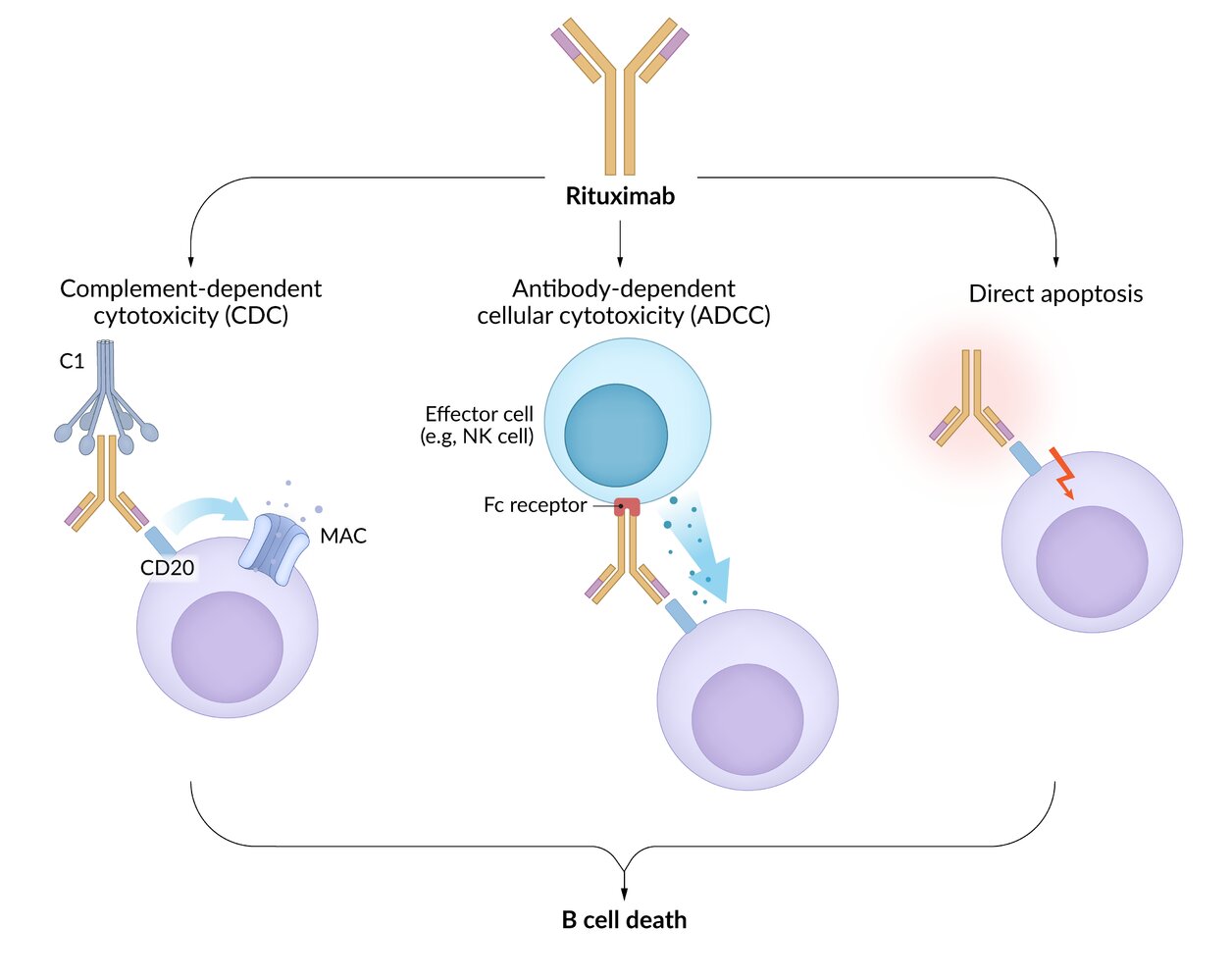
Antibody-dependent cell-mediated cytotoxicity (ADCC) [6]
- Definition: an immune response in which Fc receptor-bearing immune effector cells (e.g., NK cells, eosinophils) bind to and lyse target cells that have specific antibodies attached to their surface antigens (pathogen or tumor-derived)
-
Characteristics
- Allows innate immune cells to recognize pathogens that do not express pathogen-associated molecular patterns (PAMPs)
- Part of the immune response to parasites
-
Effector cells
- NK cells (most common): interact with IgG-coated target cells and subsequently release cytotoxic substances (perforins, granzymes)
- Eosinophils: interact with IgE-coated helminths and subsequently release major basic proteins and other cytotoxic substances
- Other: monocytes, macrophages, neutrophils
-
Mechanism
- Binding of antibodies (e.g., IgG or IgE) produced by B-cells to antigens on target cells (e.g., tumor cells, viruses, or parasites)
- Recognition and binding of the Fc portion of bound IgG or IgE by Fc receptors expressed on the surface of effector cells (e.g., CD16 Fc receptors on NK cells)
- Activation of signaling pathways in the effector cell (e.g., NK cell), leading to cytotoxic granule release
- Destruction of the target cell (e.g., via the perforin/granzyme cell death pathway)
- Clinical significance: monoclonal antibody therapy
Anticancer monoclonal antibodies (e.g., trastuzumab, rituximab) act by neutralizing extracellular targets (e.g., membrane receptors, channels) or promoting immune system recognition via ADCC by NK cells.
-
Definition
- The ability of the immune system to recognize antigens after an initial exposure and to quickly and efficiently mount an immune response in subsequent exposures.
- Allows for the conservation of a specific acquired immune response
-
Process
- Initial exposure to a foreign antigen
- Primary immune response: activation of B cells and T cells (see the sections on B cells and T cells above)
-
Formation of memory B cells and memory T cells
-
Memory B cells: specialized plasma cells that have the ability to persist for decades following the elimination of an antigen and produce high-affinity antibodies throughout their lifespan [7][8]
- Undergo proliferation, somatic hypermutation, clonal selection, and immunoglobulin class switching (see T cell‑dependentB cell activation)
- Arrested in their differentiation and persist in the marginal zone of follicles
-
Memory T cells: specialized T cells that persist following a primary immune response to an antigen and have the ability to elicit an immediate and more potent immune response in the event of subsequent exposures to the same antigen [8][9]
- Following a primary immune response, ∼ 90% of effector T cells die via apoptosis; a small fraction of the effector T cells survive to become memory T cells.
- Can be CD4+ or CD8+
- Effector memory T cells (TEM cells, CCR7 negative cells): CD4+ T cells or CD8+ T cells that persist in the circulation and peripheral tissue.
- CD4+ TEM cells produce proinflammatory cytokines (e.g., IFN-γ).
- CD8+ TEM cells contain preformed perforin granules for immediate cytotoxicity.
- Central memory T cells (CCR7 positive cells): Persist in secondary lymphoid tissue and are able to differentiate into effector memory T cells upon activation.
-
Memory B cells: specialized plasma cells that have the ability to persist for decades following the elimination of an antigen and produce high-affinity antibodies throughout their lifespan [7][8]
-
Subsequent immune response: Re-exposure to the antigen activates the memory cells.
- Memory B cells secrete high-affinity antibodies and accelerate the secondary immune response to the antigen.
- Memory T cells mature to TEM cells and trigger an immediate release of cytokines or cytotoxicity.
- Repeated exposure to the antigen leads to more efficient immune responses.
Memory cells are a large pool of antigen-specific lymphocytes that can respond faster and more efficiently than naive lymphocytes when re-exposed to the antigen. These cells form the basis for the immunologic response to vaccinations.
Immune tolerance [4][10]
The unresponsiveness of an organism’s immune system to antigens in an effort to prevent harmful over-reactivity is called immune tolerance.
Central tolerance
-
Overview
- A type of immune tolerance that occurs at an early stage of lymphoid cell development
- Ensures that T cells and B cells that recognize self-antigens (i.e., antigens expressed by the organism's own cells) are identified before they develop into fully immunocompetent cells
-
Mechanisms
- Negative selection of T cells; and B cell clonal deletion: Lymphocytes that express receptors with a high affinity for self-antigens will undergo apoptosis.
- Development of regulatory T cells (CD4+)
- Receptor editing: B-cell specificity is reprogrammed through secondary recombination of antibody genes
Peripheral tolerance
-
Overview
- A type of immune tolerance that occurs in the immune periphery (e.g., spleen, lymph nodes) following the egression of T cells and B cells from their primary lymphoid organs
- Ensures the deletion of or induction of anergy in autoreactive T and B cells that escape into the periphery
-
Mechanisms [11]
- Anergy: a lack of a normal immune response to particular antigens, cytokines, and allergens, usually due to the absence of costimulatory signals
- Deletion of autoreactive T cells through apoptosis
- Suppression: blocking activation by inducing regulatory T cells through exposure to TGF-β in peripheral tissues
- Inhibitory receptors: recognition of self-antigens prevents B-cell activation by triggering inhibitory receptors
Autoimmunity
Autoimmunity refers to an immune reaction against the body's own cells that occurs as a result of a loss of immune tolerance. Women have a disproportionately higher incidence of autoimmune diseases than men.
Presumed pathogenesis
- Autoreactive B cells are physiologically eliminated in the bone marrow, spleen, and lymph nodes.
- T cells that attack the body's own cells undergo negative selection in the thymus or undergo apoptosis in peripheral lymphoid tissues (e.g, lymph nodes, adenoids, Peyer's patches) due to a lack of stimulation.
- If the selection mechanisms fail, immune cells can attack the body's own cells, which leads to autoimmune inflammation.
Causes of autoimmune conditions
- Mostly idiopathic
-
Sometimes elicited by a previous infection (e.g., Guillain-Barré syndrome, rheumatic fever).
- The underlying pathomechanism is molecular mimicry (the antigenic resemblance between molecules on some pathogens and those of normal cells in the body)
- Persistent antigenic stimuli
- Breach of central tolerance
-
Genetic predisposition, e.g.:
- HLA-B8, e.g., myasthenia gravis, Graves disease, Addison disease
- HLA‑B27, e.g., ankylosing spondylitis, reactive arthritis, psoriatic arthritis, ulcerative colitis
- HLA-DR4, e.g., rheumatoid arthritis, type 1 diabetes, pemphigus vulgaris, Addison disease
- HLA-DR3, e.g., type 1 diabetes, SLE, Hashimoto thyroiditis, Graves disease
- HLA-DR2, e.g., SLE, Goodpasture syndrome, multiple sclerosis
- HLA‑DQ2 and HLA-DQ8, e.g., gluten‑sensitive enteropathy
- HLA-DR5, e.g., Hashimoto thyroiditis
- HLA-A3, e.g., hemochromatosis
Consequences
- The presence of autoreactive B cells results in the production of irregular antibodies, which can trigger various diseases.
- Autoantibodies can also be used as a diagnostic tool (see the table below).
- In T-cell mediated autoimmune reactions, there are usually no detectable specific antibodies (e.g., in multiple sclerosis).
Autoantibodies
| Overview of autoantibodies | ||
|---|---|---|
| Name | Target | Potentially associated conditions |
| Antinuclear antibodies (ANAs) |
|
|
| Perinuclear antineutrophil cytoplasmic antibodies (p-ANCAs, MPO-ANCAs) |
|
|
| Cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCAs, PR3-ANCAs) |
|
|
| Antithyroglobulin antibodies |
|
|
| Thyroid peroxidase antibodies (TPO Abs) |
|
|
| TSH receptor antibodies |
|
|
| Antiendomysial antibodies (EMA; IgA) |
|
|
| Transglutaminase antibodies (IgA) |
|
|
| Antigliadin antibodies (DGP IgG, DGP IgA) |
|
|
| Acetylcholine receptor antibodies |
|
|
| Anti-glomerular basement membrane antibodies |
|
|
| Anti-β2 glycoprotein antibodies |
|
|
| Anticardiolipin antibodies |
|
|
| Rheumatoid factor |
|
|
| Anti-CCP antibodies |
|
|
| Lupus anticoagulant |
|
|
| Anticentromere antibodies |
|
|
| Antidesmoglein antibodies |
|
|
| Anti-glutamic acid decarboxylase antibodies |
|
|
| Islet cell cytoplasmic antibodies |
|
|
| Antihemidesmosome antibodies |
|
|
| Antisynthetase antibodies (anti-Jo-1 antibodies) |
|
|
| Anti-SRP antibodies |
|
|
| Anti-Mi-2 antibodies (antihelicase antibodies) |
|
|
| Antimitochondrial antibodies |
|
|
| Anti-intrinsic factor antibodies |
|
|
| Anti-parietal cell antibodies |
|
|
| Anti-phospholipase A2 receptor antibodies |
|
|
| Anti-Scl-70 antibodies |
|
|
| Anti-smooth muscle antibodies |
|
|
| Anti-liver-kidney-microsomal antibodies type 1 |
|
|
| Anti-SSA (anti-Ro) antibodies Anti-SSB (anti-La) antibodies |
|
|
| Anti-presynaptic calcium channel antibodies |
|
|
| Antihistone antibodies |
|
|
| Anti-dsDNA antibodies |
|
|
| Anti-Smith antibodies |
|
|
| Anti-U1 RNP antibodies |
|
|
-
Epidemiology
- Primary immunodeficiency diseases are rare
- Approx. 1–2/1,000 general population are immune deficient.
-
Most common types
- Primary
- Fanconi anemia
- Selective IgA deficiency
- Wiskott-Aldrich syndrome
- Ataxia telangiectasia
- DiGeorge syndrome
- See “Congenital immunodeficiency disorders” for more information.
- Secondary
- HIV/AIDS
- Iatrogenic immune suppression
- Primary
-
Clinical findings
- The main symptom of primary immunodeficiency is a pathological susceptibility to infection.
- The type of susceptibility is characterized by the invading pathogen, localization, course, severity, and number of infections.
- Not all immune deficiencies are clinically apparent.
- B-cell deficiencies (decreased number of B cells and/or impaired B-cell function) typically results in recurrent bacterial infections.
- T-cell deficiencies typically result in recurrent viral and fungal infections.
| Overview of immune deficiency and infections | |||
|---|---|---|---|
| Defective immune system component | Bacteria | Viruses | Fungi/parasites |
| ↓ T cells |
|
|
|
| ↓ B cells |
|
|
|
| ↓ Complement |
|
|
|
| ↓ Granulocytes |
|
|
|
Common pathogens of recurrent infections in granulocyte-deficient individuals (Staphyloccocus, Pseudomonas aeruginosa, Nocardia, Serratia, Burkholderia cepacia): Some Pathogens Need Strong anti-Biotics.