The adrenal gland is a paired retroperitoneal organ located on the upper pole of each kidney. It receives its arterial supply from the superior, middle, and inferior suprarenal arteries and drains into the right and left suprarenal veins. The lymphatics drain into the left aortic and the right caval lymph nodes. The adrenal gland has two layers: the adrenal cortex (outer layer), which is derived from the mesoderm, and the adrenal medulla (inner layer), which is derived from neural crest cells. The adrenal medulla is composed of chromaffin cells, which secrete catecholamines (norepinephrine, epinephrine, dopamine). The adrenal cortex consists of three layers: the zona glomerulosa, the zona fasciculata, and the zona reticularis, which are responsible for the synthesis of mineralocorticoids, glucocorticoids, and androgens (precursors for estrogen and testosterone), respectively. Mineralocorticoids regulate renal sodium and water reabsorption and potassium excretion, while glucocorticoids play an important role in glucose metabolism. Diseases of the adrenal glands include adrenal insufficiency (due to an infection, hemorrhage, or autoimmune destruction), hyperaldosteronism (due to hyperplasia or adenoma), and hypercortisolism (due to hyperplasia, adenoma, or exogenous administration).
Overview
- Description: : a bilateral endocrine gland composed of an outer cortex that produces steroid hormones and inner medulla that produces catecholamines (e.g., epinephrine).
-
Morphology
- Height and thickness: ∼ 5 cm
- Width: 1–2 cm
- The left adrenal gland is shaped like a crescent, while the right resembles a pyramid. [1]
-
Location
- Primary retroperitoneal organs
- Each gland is located superior to the upper pole of the kidney.
- Enclosed by the renal fascia and adipose capsule of the kidney
-
Embryology [2]
- Adrenal cortex: derived from mesothelial cells
- Adrenal medulla: derived from chromaffin cells, which originate in the neural crest and migrate to the paraganglia and adrenal medulla during embryonic development
Function
- Adrenal cortex: production of steroid hormones (mineralocorticoids, glucocorticoids, and androgens)
- Adrenal medulla: production of catecholamines (epinephrine, norepinephrine)
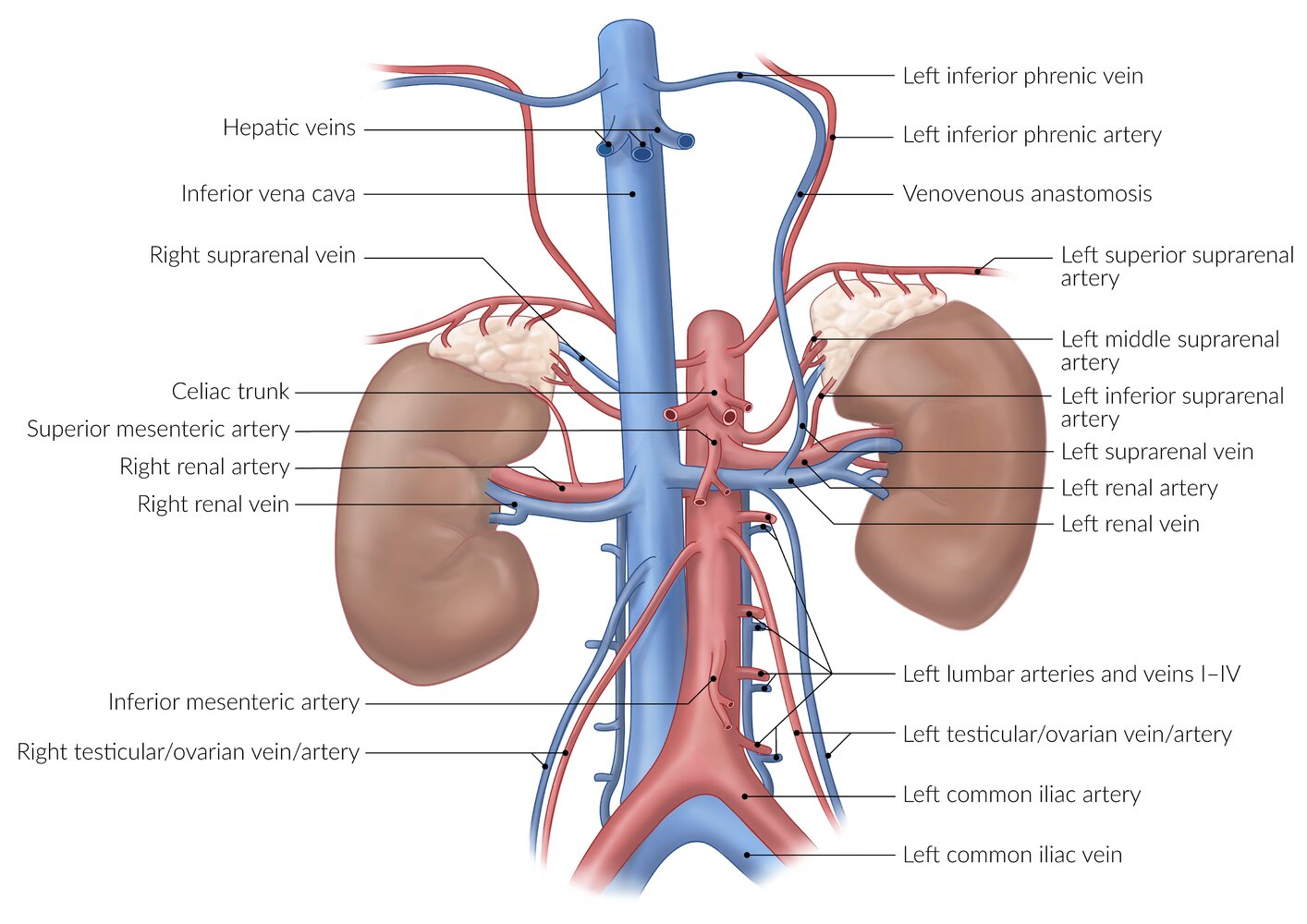
Blood supply and lymphatics
Because the adrenal glands produce and subsequently release a number of essential hormones, they are very well vascularized and perfused.
-
Arterial blood supply
- Superior suprarenal artery
- Middle suprarenal artery
- Inferior suprarenal artery
-
Venous drainage
- The right suprarenal vein drains into the inferior vena cava.
- The left suprarenal vein drains into the left renal vein and then into the inferior vena cava.
-
Lymphatic drainage
- Left aortic lymph nodes
- Right caval lymph nodes
Venous drainage is different for the left and right adrenal gland. The left suprarenal vein empties into the left renal vein, while the right suprarenal vein drains into the inferior vena cava.
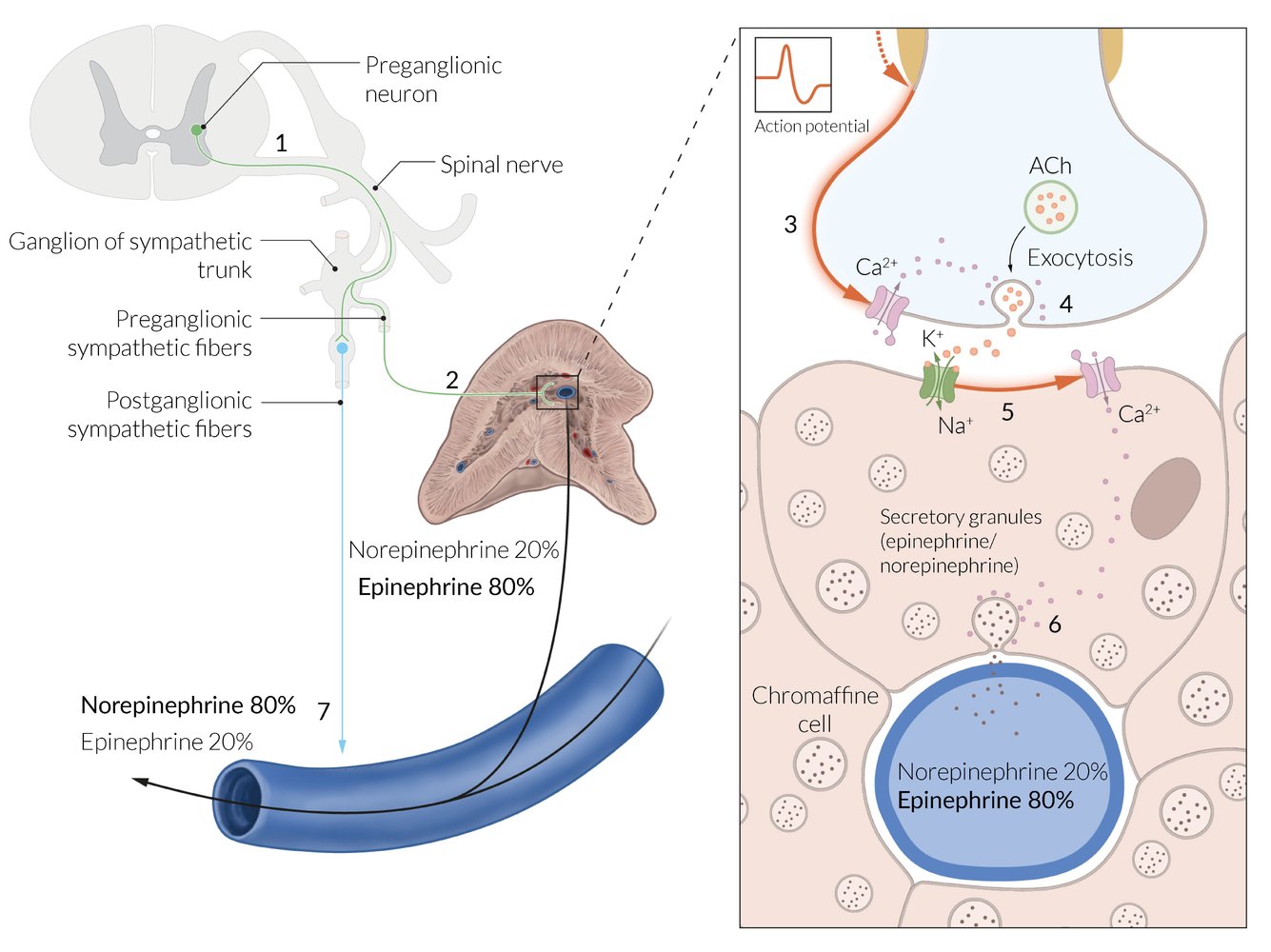
Innervation [3]
- Sympathetic: preganglionic sympathetic fibers from the major and minor splanchnic nerves
- Parasympathetic: fibers from phrenic and vagal nerve
Adrenal cortex
- Overview: The adrenal cortex is surrounded by a fibrous capsule and composed of three main zones (or layers).
-
Zones
-
Zona glomerulosa
- Outermost layer
- Cells are arranged in oval clusters surrounded by connective tissue from the fibrous capsule.
- Main site of aldosterone production
-
Zona fasciculata
- Located between the zona glomerulosa and zona reticularis
- Cells are arranged in straight columns that are separated by small fibrous septa.
- Main site of glucocorticoid production
-
Zona reticularis
- Inner cortical layer
- Small cells are arranged in an irregular netlike formation surrounded by connective tissue and capillaries.
- Main site of androgen production
-
Zona glomerulosa
To remember the microscopic anatomy and functions of the adrenal cortex going from outside to inside, think “GFR, the deeper you go, the sweeter it gets: Salt (aldosterone, zona Glomerulosa), Sugar (glucocorticoids, zona Fasciculata), and Sex (androgens, zona Reticularis).”
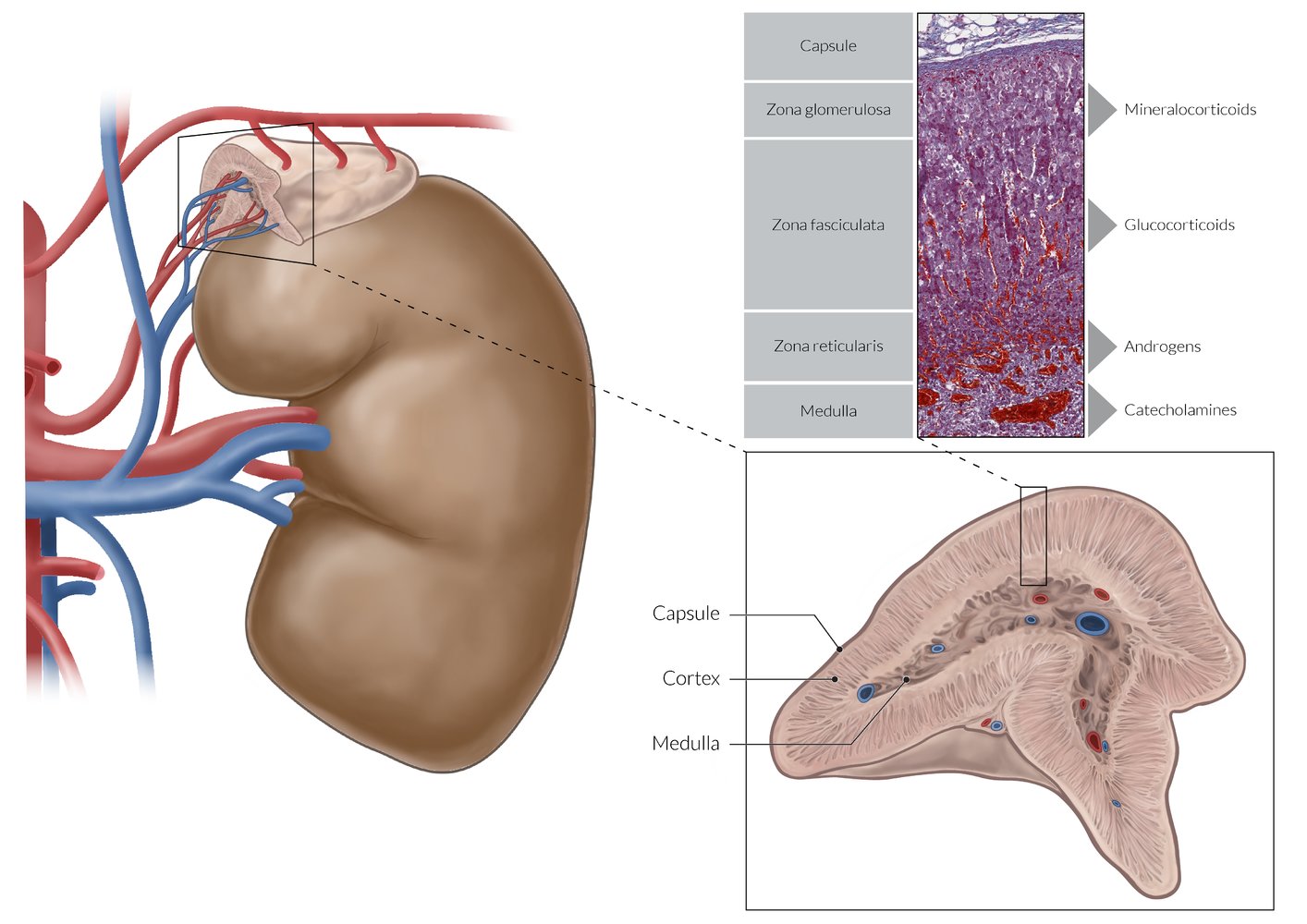
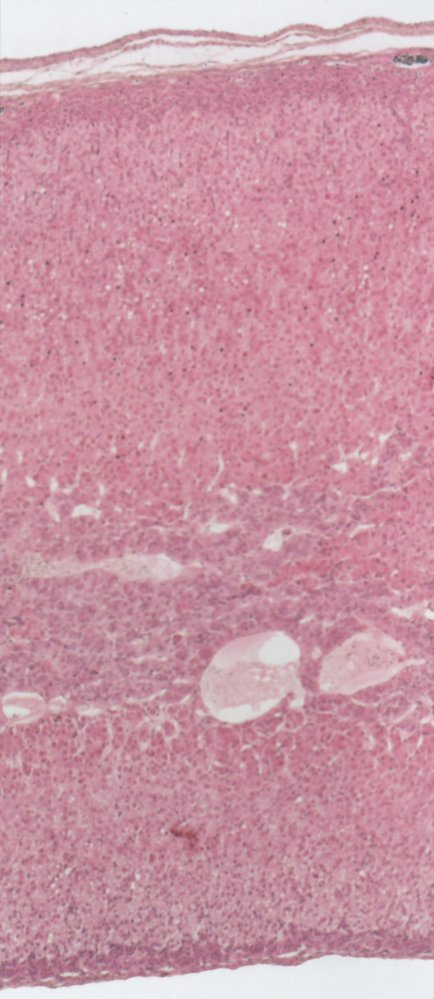
Adrenal medulla
- Overview: : The adrenal medulla is surrounded by the adrenal cortex and made up of modified sympathetic postganglionic neurons. [4]
-
Organization
- Contains large, irregularly shaped, chromaffin cells with many secretory granules for catecholamine storage
- Arranged in clusters and grouped around fenestrated capillaries
- Tumors originating from chromaffin cells are called pheochromocytomas.
The cells of the adrenal medulla are modified sympathetic cells that are controlled by cholinergic synapses.

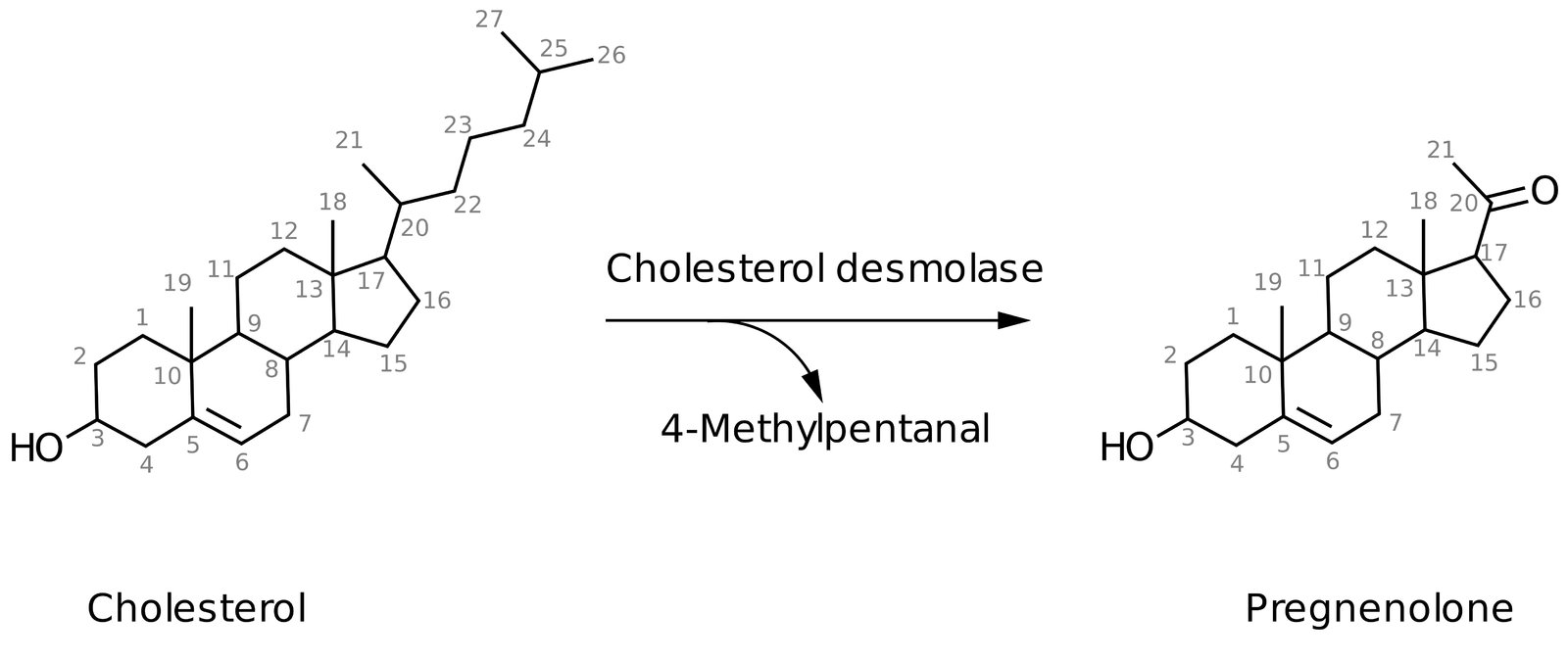
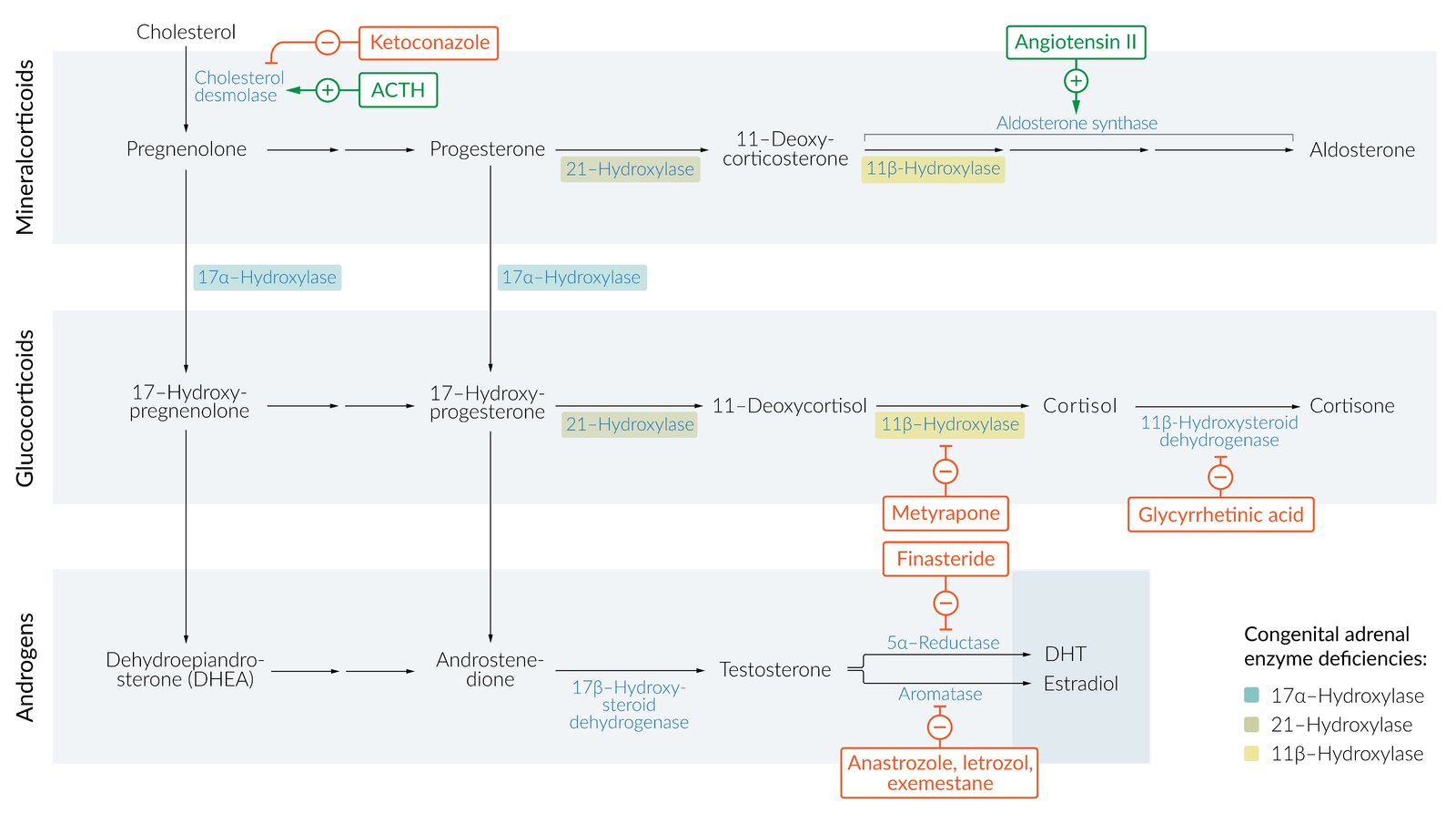
Synthesis of all steroid hormones begins with the common precursor molecule, cholesterol, which is converted into pregnenolone via cholesterol desmolase. Cholesterol desmolase can be inhibited by azole antifungals.
| Overview of the hormones of the adrenal cortex | |||
|---|---|---|---|
| Features | Aldosterone | Cortisol | Dehydroepiandrosterone (DHEA) |
| Hormone class |
|
|
|
| Production site |
|
|
|
| Function |
|
|
|
| Regulation of secretion |
|
|
|
| Associated disorders |
|
|
|
RAAS regulates the release of mineralocorticoids.
Mineralocorticoid synthesis
| Biosynthesis of aldosterone | |||
|---|---|---|---|
| Steps | Precursor | Enzyme | Product |
| 1. |
|
|
|
| 2. |
|
|
|
| 3. |
|
|
|
| 4. |
|
|
|

Mineralocorticoid function
-
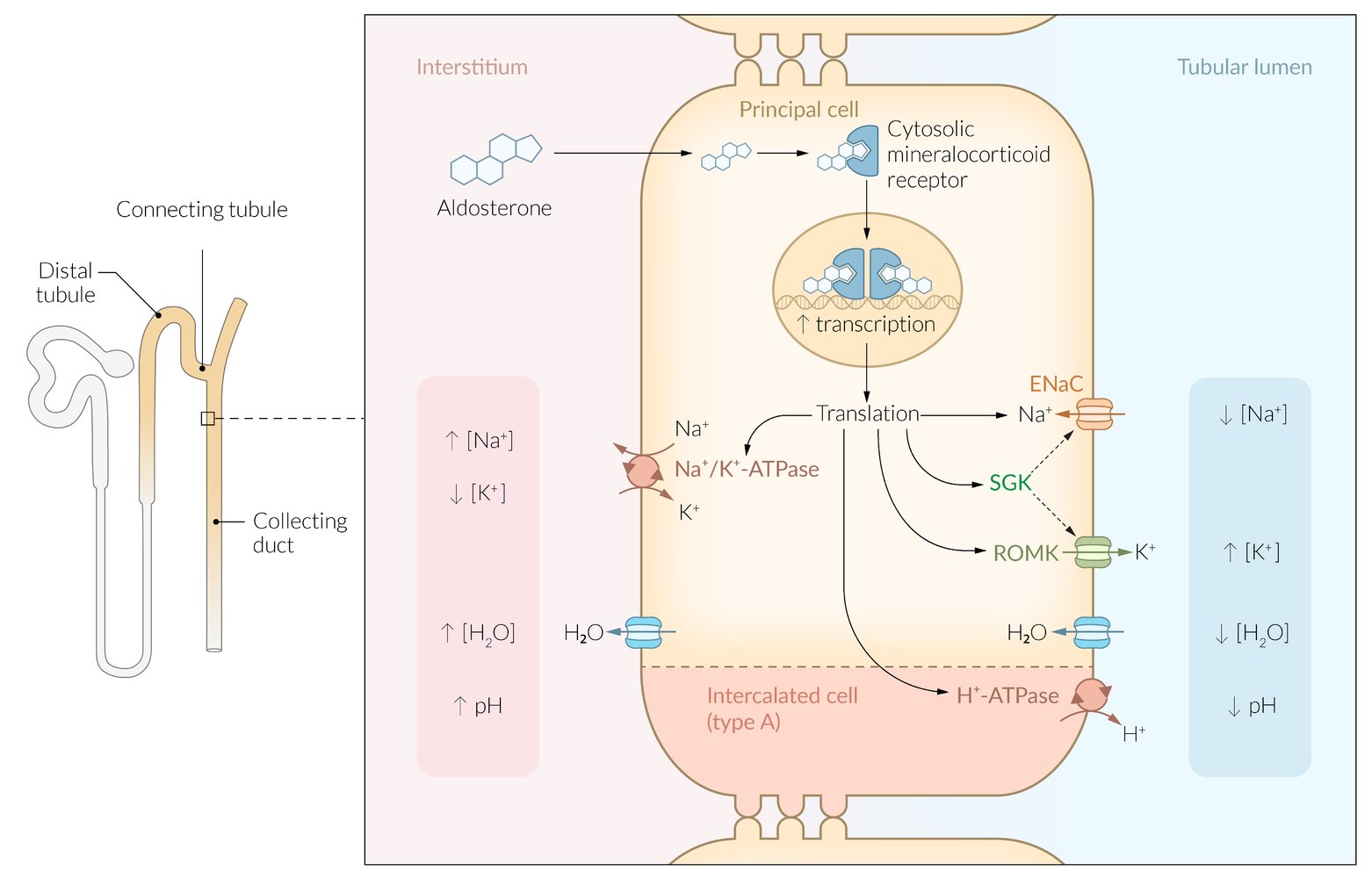
Mechanism of action: aldosterone binds to intracellular mineralocorticoidreceptors in the distal tubule and collecting duct of the kidney, inducing protein synthesis and following changes:
- ↑ Na+/K+-ATPase in the basolateral membrane → transportation of Na+ out and K+ into the tubule cells
- ↑ Apical H+-ATPase → H+ excretion
- ↑ Na+ channels (ENaC; epithelial sodium channel) → ↑ Na+ reabsorption
- ↑ K+ channels (ROMK; renal outer medullary potassium channel) in the luminal membrane → ↑ K+ excretion
-
Effects
- These aldosterone-induced changes produce a concentration gradient →Na+ reabsorption → water reabsorption; and K+ secretion into the urine.
- Ultimately, these effects →↑ blood pressure, hypokalemia, and ↑ pH level.
Aldosterone stimulates potassium excretion in the collecting duct of the kidney as well as sodium and water retention.

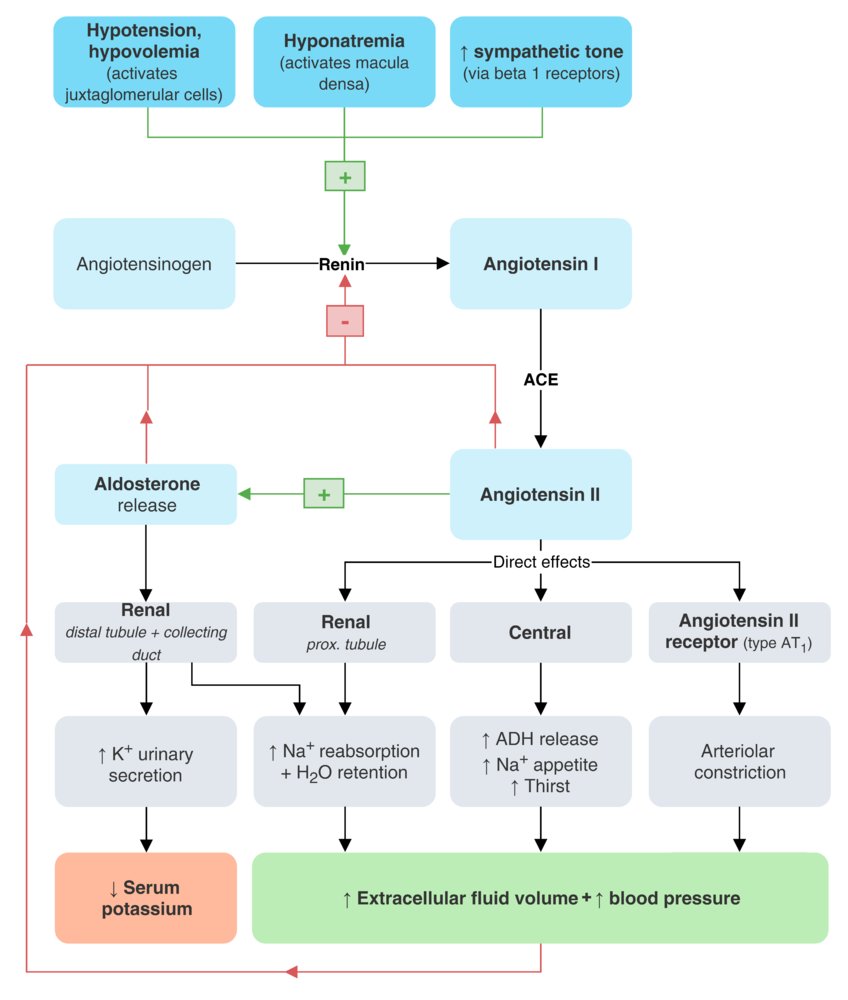
Renin-angiotensin-aldosterone system (RAAS)
-
Overview
- Hormone system that regulates blood pressure, electrolyte homeostasis, and fluid balance
- Important target for blood pressure medication (e.g., ACE inhibitors, AT1 blockers, diuretics) and involved in the development of cardiac conditions (e.g., congestive heart failure, cardiac remodeling)
-
Feedback mechanism
- A stimulus triggers renin secretion.
- Renin promotes the conversion of angiotensinogen (produced in the liver) to angiotensin I (AT I).
- AT I is turned into angiotensin II via angiotensin-converting enzyme (highest concentration in the lungs where it is produced by vascular endothelial cells).
- Angiotensin II causes vasoconstriction and triggers the secretion of aldosterone.
-
Regulation of secretion
- Positive feedback
- ↓ Renalperfusion (e.g., due to hypotension, stimulation of β1 receptors in the kidney) triggers renin release.
- ↑ Serum potassium concentration → stimulation of zona glomerulosa cells → ↑ secretion of aldosterone
- Negative feedback: ↑ systemic arterial blood pressure → ANP release from atrial myocytes → inhibition of renin release → vasodilation, natriuresis, and ↑ diuresis (see ”Atrial natriuretic peptide”) [5]
- Positive feedback
Clinical significance
- Primary adrenal insufficiency
- Congenital adrenal hyperplasia
- Primary hyperaldosteronism (Conn syndrome) and secondary hyperaldosteronism
- Hyperkalemic renal tubular acidosis (type 4)
Glucocorticoid synthesis
| Biosynthesis of cortisol | ||||
|---|---|---|---|---|
| Steps | Precursor | Enzyme | Product | |
| 1. | Pregnenolone pathway |
|
|
|
| Progesterone pathway |
|
|
|
|
| 2. | Pregnenolone pathway |
|
|
|
| Progesterone pathway |
|
|
||
| 3. | Common pathway |
|
|
|
| 4. | Common pathway |
|
|
|

Glucocorticoid function
-
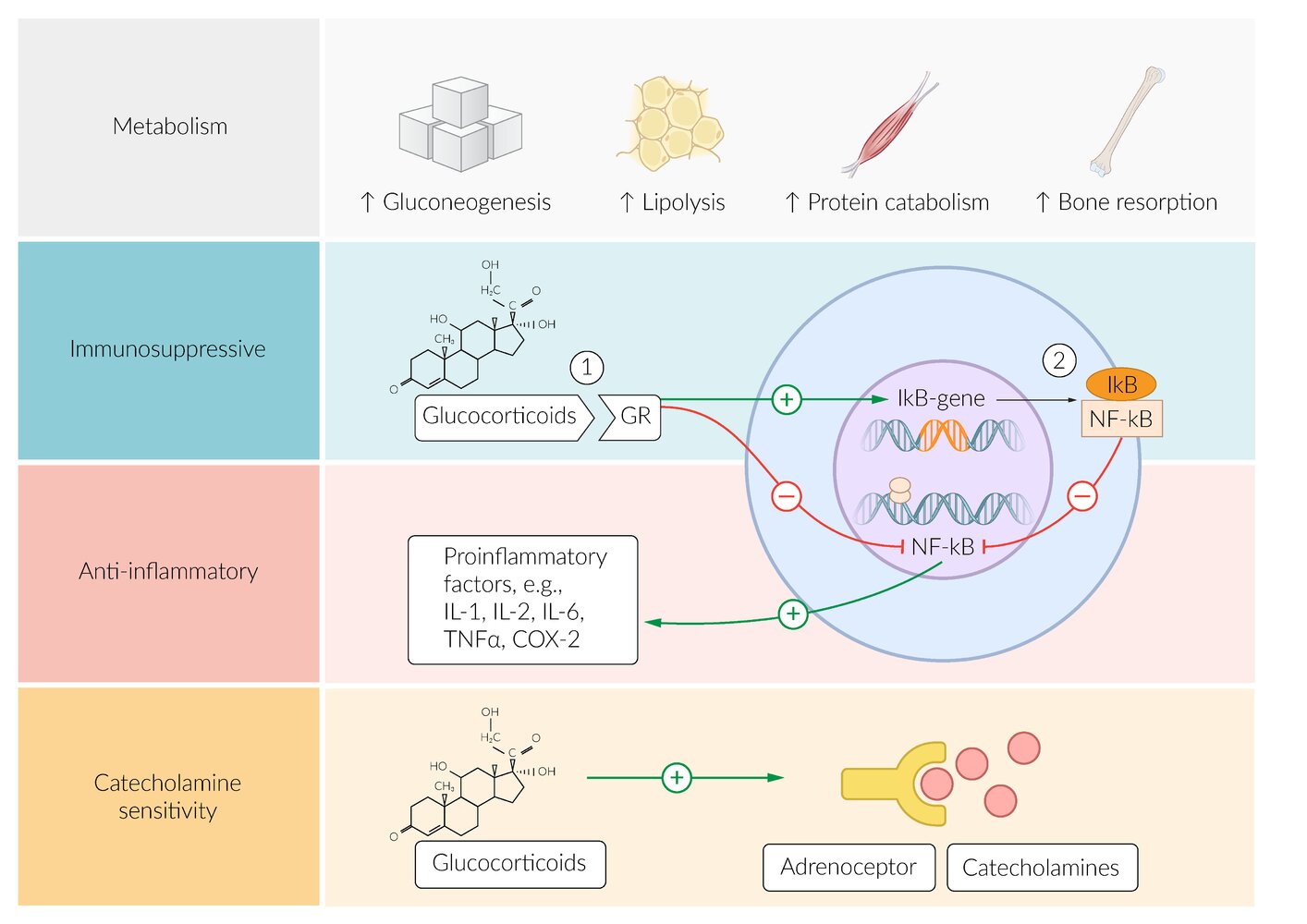
Metabolism: Cortisol plays an important role in the mobilization of energy reserves.
- ↑ Gluconeogenesis to maintain blood glucose levels
- ↑ Glycogen synthesis to maintain glucose storage
- ↑ Protein catabolism
- ↑ Lipolysis
- ↑ Appetite
- ↑ Insulin resistance
- Immune system: antiinflammatory and immunosuppressive effects (see “Pharmacodynamics of glucocorticoids”)
- Wound healing: fibroblast inhibition → ↓ collagen synthesis → ↓ wound healing
- Blood pressure: mild mineralocorticoid effect (stimulation of aldosterone receptors in high concentrations) and ↑ potassium excretion → ↑ blood pressure
See “Side effects of glucocorticoid therapy.”
To remember the effects of cortisol, think “A BIG FIB”: increased Appetite, Blood pressure, Insulin resistance, Glucose production, and decreased Fibroblasts, Immunity, and Bone formation.
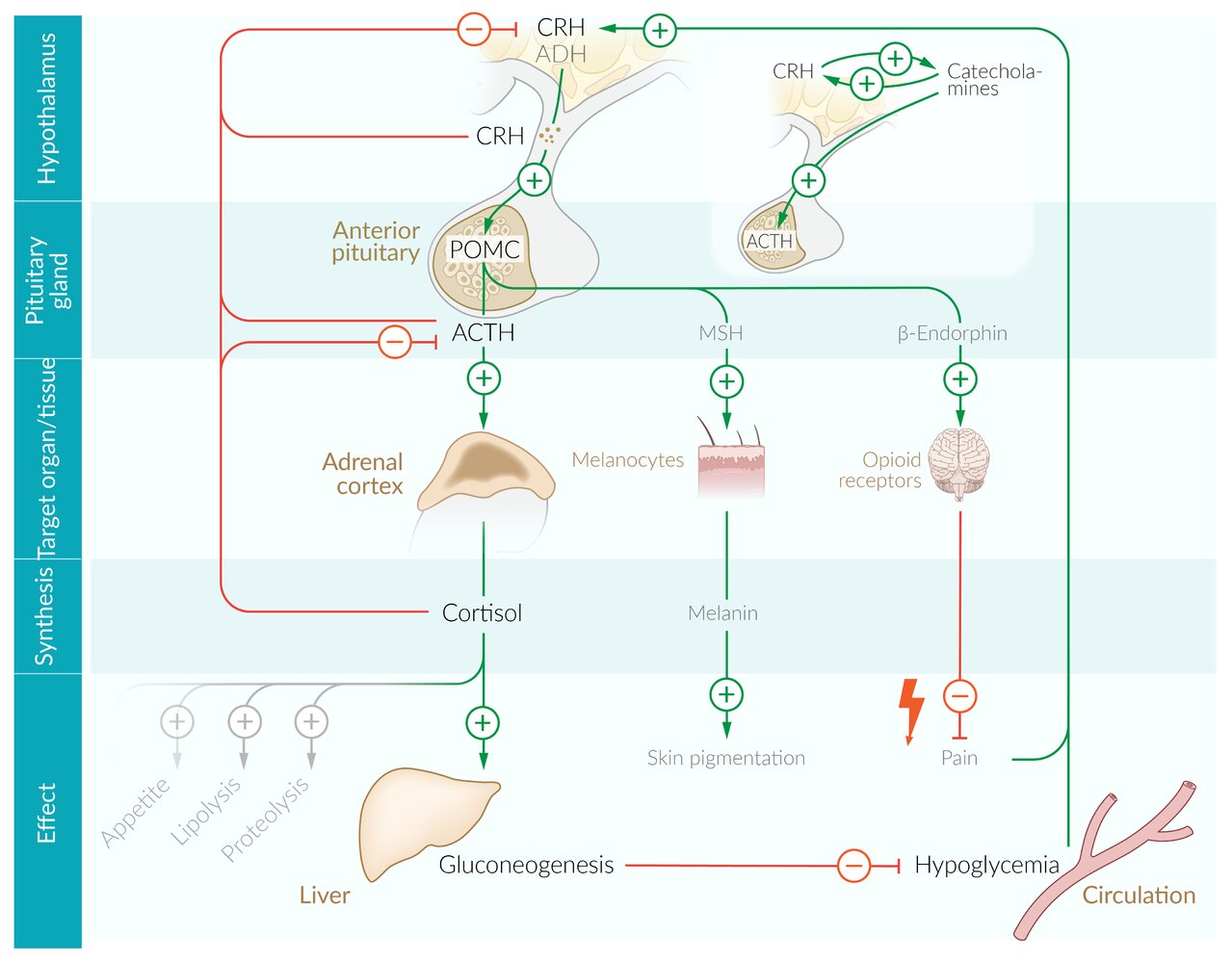
Hypothalamic-pituitary gland-adrenal cortex
- Feedback mechanism: a stimulus causes increased secretion of corticotropin-releasing hormone (CRH) → ↑ secretion of adrenocorticotropic hormone (ACTH) in the pituitary gland → ↑ secretion of glucocorticoids in the adrenal cortex.
-
Regulation of secretion
-
Positive feedback: A number of stimuli can trigger CRH release.
- Psychological/physical pain and stress
- Pyrogens, epinephrine, histamine
- Hypoglycemia
- Hypotension
- Negative feedback: Glucocorticoids themselves trigger a negative feedback loop that inhibits the secretion of CRH and ACTH.
-
Circadian rhythm [6]
- Endogenous biological rhythm influences CRH secretion.
- Cortisol levels are highest early in the morning and decrease during the day, until they drop sharply during the night and the early phase of sleep.
-
Positive feedback: A number of stimuli can trigger CRH release.
Cortisol inhibits the secretion of CRH and ACTH via negative feedback, which, in turn, results in a decrease in cortisol secretion.
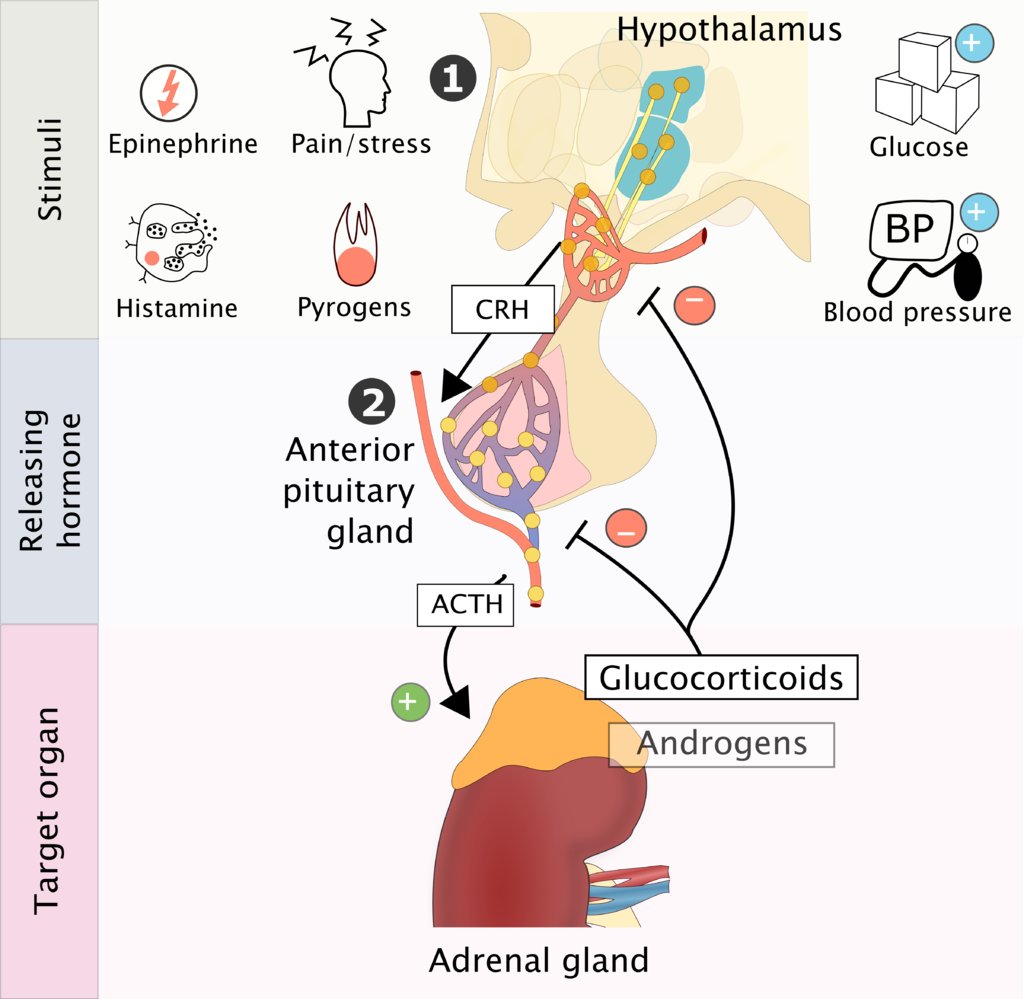

Clinical significance
- Adrenal insufficiency
- Congenital adrenal hyperplasia
- Hypocortisolism
- Hypercortisolism
Androgen synthesis [7]
| Biosynthesis of androgens | |||||||
|---|---|---|---|---|---|---|---|
| Steps | Precursor | Enzyme | Product | ||||
| 1. | Pregnenolone pathway |
|
|
|
|||
| Progesterone pathway |
|
|
|||||
| 2. | Pregnenolone pathway |
|
|
|
|||
| Progesterone pathway |
|
|
|||||
| 3. | Pregnenolone pathway |
|
|
|
|||
- Further processing occurs in the target tissue: gonads, brain, adipose tissue , skin, bone, and placenta (see “Function” below).
- 17α-hydroxylase defect causes a rare form of CAH involving pseudohermaphroditism in males and delayed puberty in females.
In both men and women, DHEA and androstenedione are produced in the adrenal cortex, which are precursors for testosterone and estrogen. Testosterone is produced by Leydig cells in the testes in men and, to a lesser degree, ovarian stroma in women.
AnDRostenedione comes from the ADRenal glands and TESTostetone from the TESTes.

Androgen function
Adrenal androgensDHEA and androstenedione serve as precursors of:
-
Androgens
- Androstenedione is converted into testosterone, which is then transformed into dihydrotestosterone (DHT) via 5α-reductase (defect leads to 5α-reductase deficiency).
- 5α-reductase inhibitors (e.g., finasteride) inhibit the conversion and are used in the treatment of BPH.
-
Estrogen
- Aromatase converts testosterone into estradiol and androstenedione, which is then transformed into estrone.
- Synthesized in men and postmenopausal women
Effects of androgens
-
Androgens influence male sexual differentiation during embryonic development.
-
Testosterone stimulates the differentiation of the following structures:
- Epididymis
- Vas deferens
- Seminal vesicles
-
DHT stimulates the differentiation of:
- Prostate
- Penis
- Scrotum
-
Testosterone stimulates the differentiation of the following structures:
- Male pubertal development of secondary sexual characteristics; (e.g., growth spurt, increased muscle mass, penile growth, deepening of the voice,Adam's apple growth, acne)
- Spermatogenesis
- Increased libido
- Anabolic effects on muscles and bones (epiphyseal plates closure due to increased conversion to estrogen)
- Increased secretion of sebaceous glands
- Stimulate erythropoiesis (↑ RBCs)
- Influence behavior
Effects of estrogen
- Female sexual differentiation during embryonic development
- Female pubertal development of secondary sexual characteristics
- See “Estrogen and associated diseases.”
Generally, the effects of androgens in women become apparent only in cases of androgen excess (e.g., PCOS, androgen-secreting tumors).
Hypothalamic-pituitary gland-adrenal cortex feedback mechanism
- A stimulus triggers increased secretion of CRH → ↑ secretion of ACTH in the pituitary gland → ↑ secretion of androgens in the adrenal cortex.
Clinical significance
- Adrenal insufficiency
- Congenital adrenal hyperplasia
- Androgen-secreting tumors
Catecholamine synthesis
Catecholamines (norepinephrine, epinephrine, dopamine) can also be synthesized at sites in the human body other than the adrenal medulla, such as specific regions of the CNS; and postganglionic adrenergic neurons.
| Biosynthesis of catecholamines | |||||
|---|---|---|---|---|---|
| Step | Precursor | Enzyme | Cofactor | Product | |
| First hydroxylation |
|
|
|
|
|
| Second hydroxylation |
|
|
|
||
| Decarboxylation |
|
|
|
|
|
| Hydroxylation of the β-C-Atom |
|
|
|
|
|
| Methylation |
|
|
|
|
|
Epinephrine has the shortest half-life of the catecholamines.
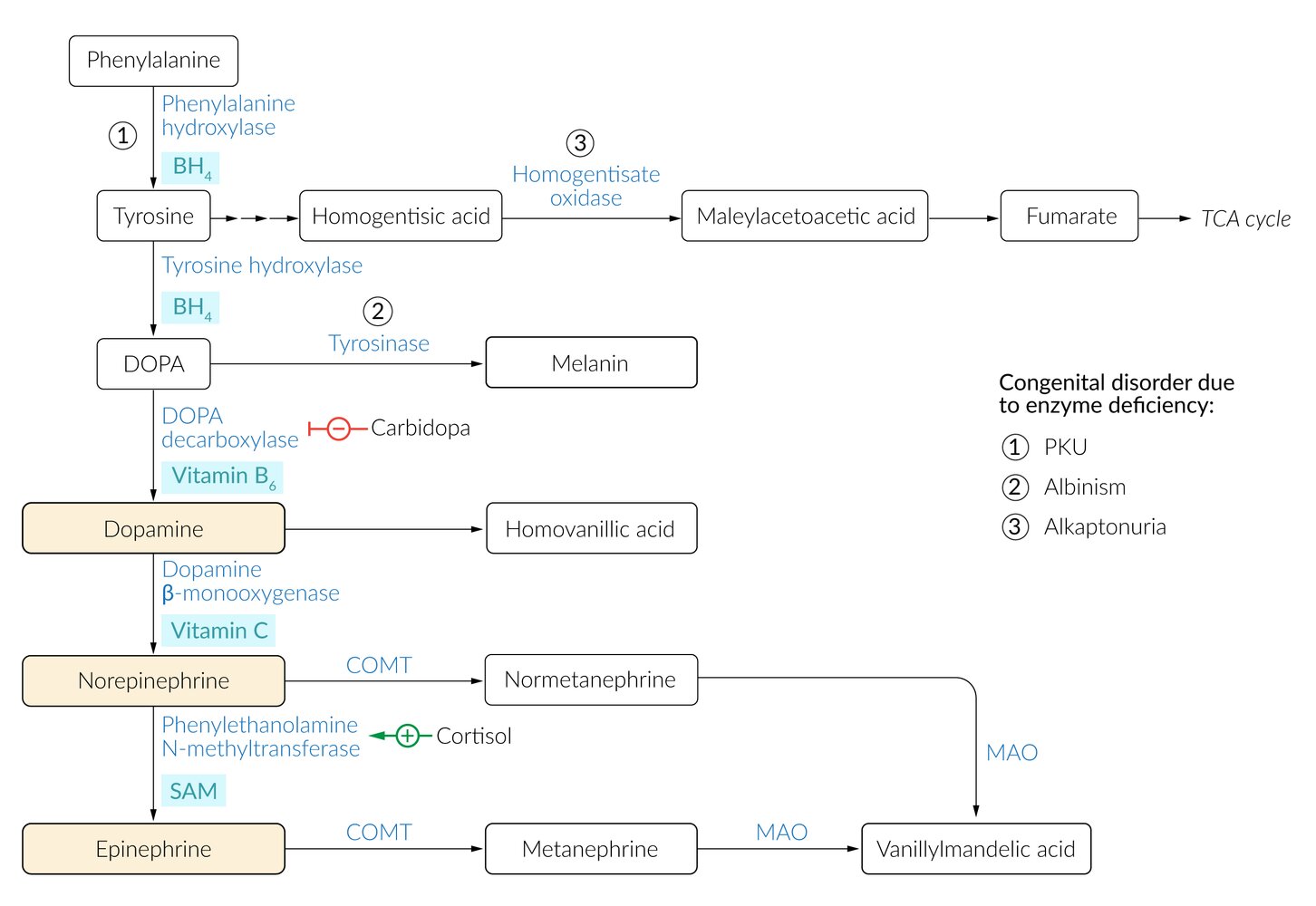
Catecholamine function
- Mechanism: Catecholamines bind to various adrenergic receptors (see table below) located on different organs and tissues.
-
Effects
- Binding triggers tissue-specific responses.
- This leads to sympathetic activation to prepare the human body for a fight-or-flight reaction.
| Overview of peripheral adrenergic receptors | ||||
|---|---|---|---|---|
| Receptor | G protein | Signal transduction | Location | Effect |
| α1 |
|
|
|
|
|
|
|||
| α2 |
|
|
|
|
|
|
|||
|
|
|||
| β1 |
|
|
|
|
|
|
|||
| β2 |
|
|
||
|
|
|||
|
|
|||
|
|
|||
|
|
|||
| β3 |
|
|
||
Regulation of secretion
- Catecholamine secretion can be triggered by a number of stimuli, such as high-stress situations (e.g., fight-or-flight) or cortisol.
Catecholamine degradation
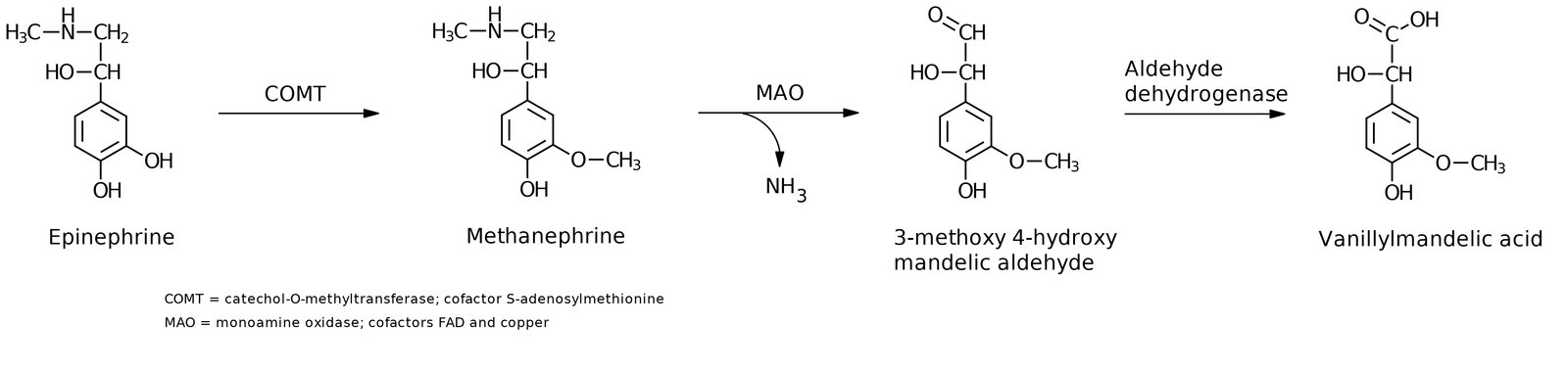
- Enzymatic degradation occurs via catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO).
- MAO can be blocked by MAO inhibitors to elevate catecholamine concentration in synaptic cleft.
- 3,4-Dihydroxyphenylacetic acid (DOPAC) is a neuronal metabolite formed by the breakdown of dopamine by monoamine oxidase.
- Vanillylmandelic acid (VMA) is an end-stage metabolite. Urinary excretion is elevated in patients with pheochromocytoma and neuroblastoma.
Clinical significance
- Pheochromocytoma
- Neuroblastoma
- Hyperphenylalaninemia and phenylketonuria