Introduction
The adrenal glands are small, triangle-shaped endocrine organs that are located on top of each kidney that play a crucial role in metabolism, fluid and electrolyte balance, and stress response modulation. This article focuses on normal adrenal function. Pathologic adrenal conditions are discussed in detail in separate articles.
Anatomy
The adrenal glands are bilateral structures each weighing about 4-6 g in adults. They are composed of 2 distinct regions: the adrenal cortex and the adrenal medulla.
Adrenal cortex
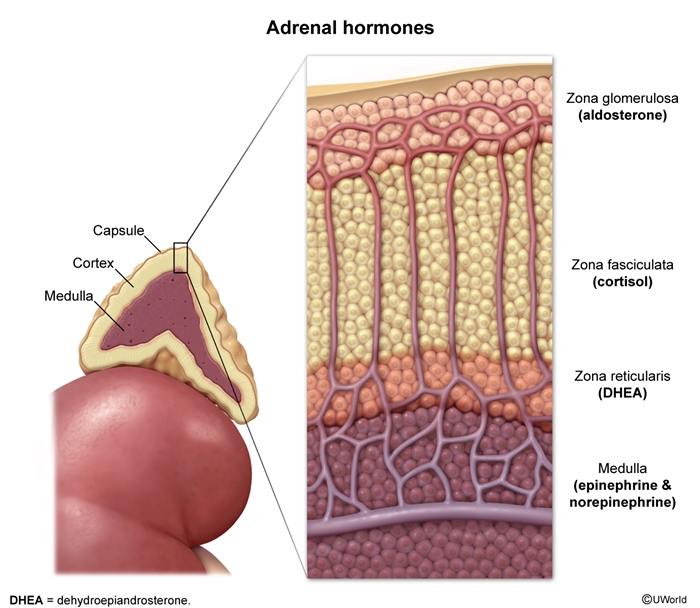
The outer portion of the gland comprises about 80%-90% of its mass. It is further divided into 3 zones:
- Zona glomerulosa: The outermost layer, responsible for mineralocorticoid production (primarily aldosterone)
- Zona fasciculata: The middle and largest layer, responsible for glucocorticoid production (primarily cortisol)
- Zona reticularis: The innermost layer, responsible for androgen production (primarily dehydroepiandrosterone [DHEA] and androstenedione)
Adrenal medulla
The inner portion of the gland, comprising about 10%-20% of its mass. It is responsible for catecholamine production (primarily epinephrine and norepinephrine.
The adrenal glands are highly vascularized, receiving blood supply from the superior, middle, and inferior adrenal arteries. Venous drainage occurs primarily through the central adrenal vein.
Physiology
The adrenal glands produce several hormones essential for maintaining homeostasis:
Glucocorticoids (primarily cortisol)
- Regulate metabolism, immune function, and stress response
- Increase blood glucose levels through gluconeogenesis and glycogenolysis
- Suppress inflammation and immune responses
- Maintain vascular tone and cardiac contractility
Mineralocorticoids (primarily aldosterone)
- Regulate electrolyte balance and blood pressure
- Promote sodium reabsorption and potassium excretion in the kidneys
- Increase blood volume and blood pressure
Adrenal androgens (DHEA, androstenedione)
- Contribute to sexual characteristics in women (in men, testosterone from testicular secretion is the main androgen)
- Serve as precursors for other sex hormones
Catecholamines (epinephrine, norepinephrine)
- Mediate the "fight or flight" response
- Increase heart rate, blood pressure, and respiratory rate
- Promote glucose mobilization and lipolysis
A summary of adrenal anatomy and physiology is shown
Adrenal regulation
The production and secretion of adrenal hormones are tightly regulated by various mechanisms:
Hypothalamic-pituitary-adrenal (HPA) axis
The HPA axis controls glucocorticoid production
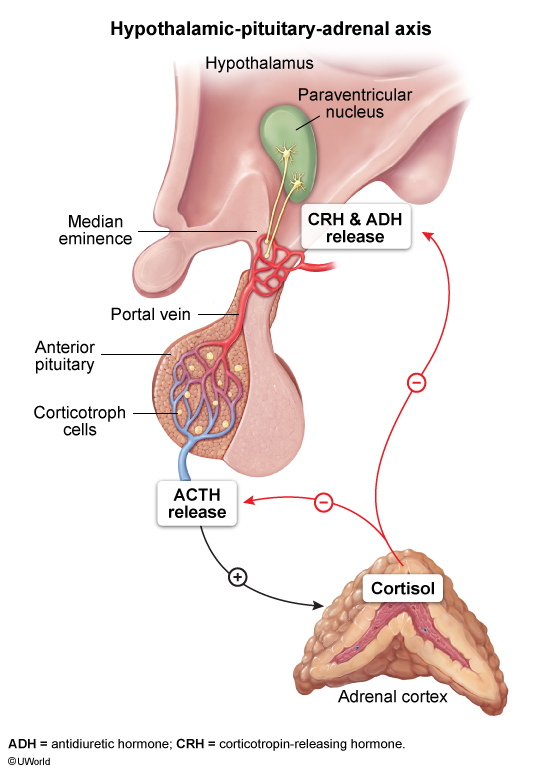
- Stress or circadian rhythms stimulate the hypothalamus to release corticotropin-releasing hormone (CRH.
- CRH stimulates the anterior pituitary to secrete adrenocorticotropic hormone (ACTH.
- ACTH stimulates the adrenal cortex to produce cortisol. Steroidogenesis is described in more detail in the section below.
- Cortisol exerts negative feedback on the hypothalamus and pituitary.
Renin-angiotensin-aldosterone system (RAAS)
RAAS regulates mineralocorticoid production
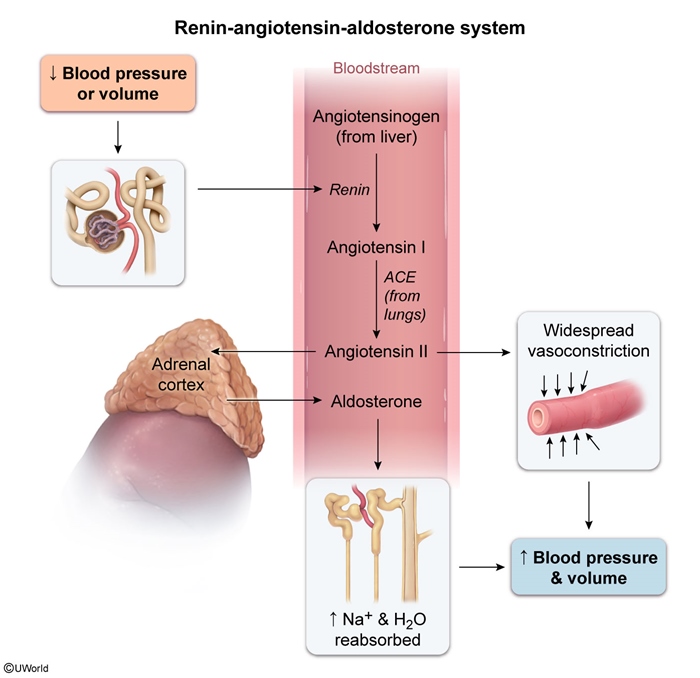
- Decreased blood volume or pressure stimulates renin release from the kidneys.
- Renin converts angiotensinogen to angiotensin I, which is then converted to angiotensin II.
- Angiotensin II stimulates aldosterone production in the zona glomerulosa.
Sympathetic nervous system
The sympathetic nervous system controls catecholamine production in the adrenal medulla
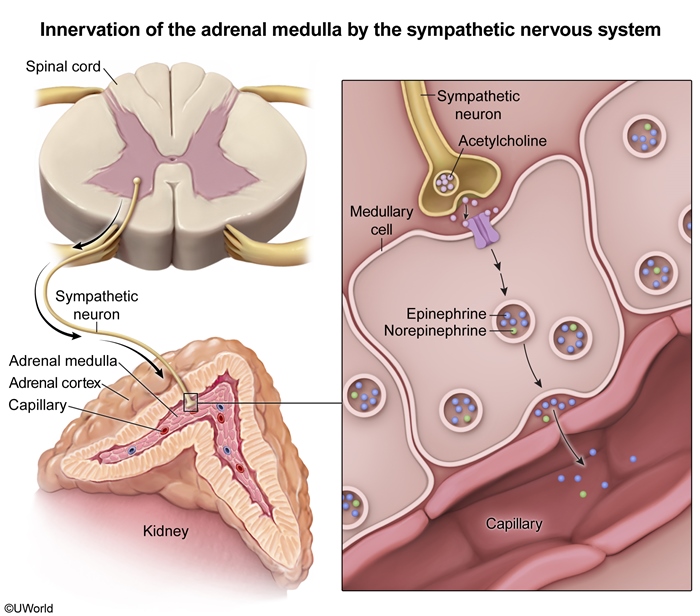
- Sympathetic stimulation triggers the release of epinephrine and norepinephrine.
ACTH and steroidogenesis
ACTH mechanism of action
ACTH acts by binding to the melanocortin 2 receptor on adrenocortical cells, activating adenylyl cyclase. This increases cyclic AMP, stimulating protein kinase A and phosphorylation of various proteins. ACTH has both acute and chronic effects on steroidogenesis:
- Acute effects (within minutes):
- Increases cholesterol transport from the cytoplasm to the inner mitochondrial membrane
- Stimulates the rate-limiting step in steroidogenesis through increased activity of steroidogenic acute regulatory (StAR) protein
- Chronic effects (hours to days):
- Increases synthesis of steroidogenic enzymes
- Promotes general adrenocortical cell growth (hyperplasia) and RNA and protein synthesis
Key steps in steroid hormone synthesis pathway
In response to ACTH and/or angiotensin II, the adrenal cortex synthesizes various steroid hormones from cholesterol through a series of enzymatic reactions

- Cholesterol uptake and transport:
- Cholesterol is obtained from circulation (primarily LDL and HDL) or synthesized de novo.
- StAR protein mediates the rate-limiting step of transporting cholesterol to the inner mitochondrial membrane.
- Pregnenolone synthesis:
- Cholesterol side-chain cleavage enzyme converts cholesterol to pregnenolone.
- Zone-specific steroidogenesis:
- Zona glomerulosa (aldosterone pathway):
- Pregnenolone is converted to progesterone.
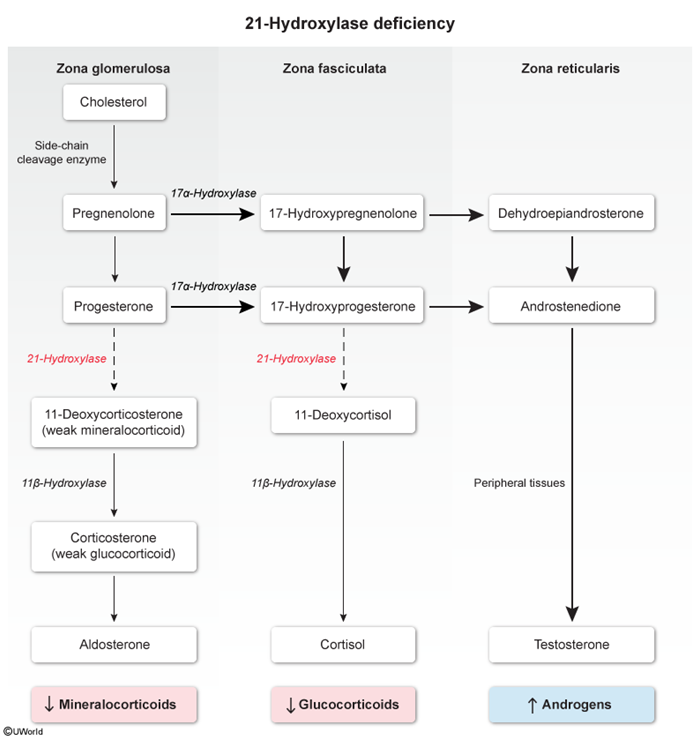
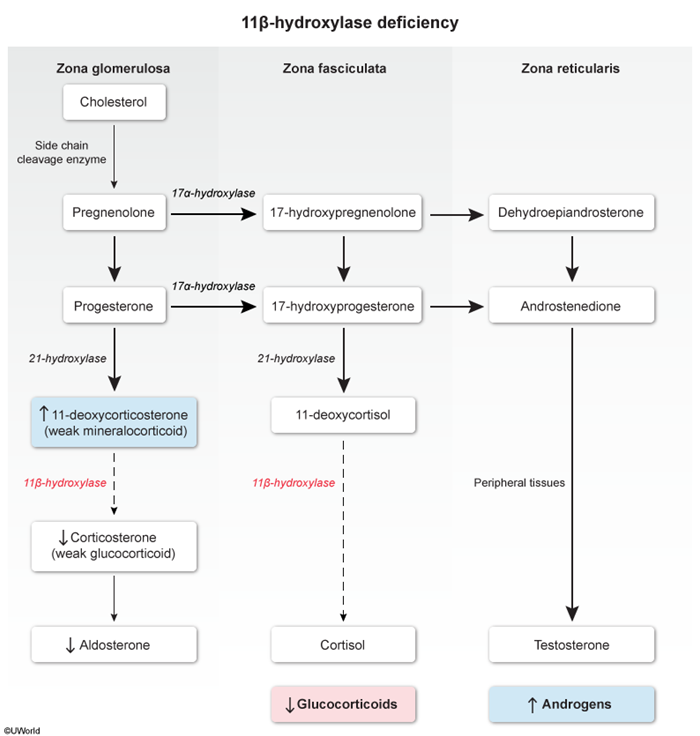
- 21-hydroxylase converts progesterone to 11-deoxycorticosterone (a weak mineralocorticoid.
- 11β-hydroxylase converts 11-deoxycorticosterone to corticosterone (a weak glucocorticoid.
- Corticosterone is converted into aldosterone.
- Zona fasciculata (cortisol pathway):
- 17α-hydroxylase converts pregnenolone to 17-hydroxypregnenolone and progesterone to 17α-hydroxyprogesterone.
- 21-hydroxylase converts 17α-hydroxyprogesterone to 11-deoxycortisol.
- 11β-hydroxylase converts 11-deoxycortisol to cortisol.
- Zona reticularis (androgen pathway):
- DHEA is converted into androstenedione.
- Peripheral tissue enzymes convert androstenedione to small amounts of testosterone.
- Zona glomerulosa (aldosterone pathway):
Certain enzyme deficiencies, such as 21-hydroxylase deficiency
Summary
The adrenal glands contain an outer layer (cortex that synthesizes glucocorticoids (cortisol), mineralocorticoids (aldosterone), and some androgens (DHEA), and an inner layer (medulla that secretes catecholamines (epinephrine and norepinephrine. The production and secretion of adrenal hormones are tightly regulated by various mechanisms. The hypothalamic-pituitary-adrenal axis controls glucocorticoid production through a cascade of hormonal signals involving CRH, ACTH, and cortisol. RAAS regulates mineralocorticoid production in response to changes in blood volume and pressure. The sympathetic nervous system governs catecholamine release from the adrenal medulla.