Chemotherapeutic agents, also referred to as antineoplastic agents, are used to directly or indirectly inhibit the uncontrolled growth and proliferation of cancer cells. They are classified according to their mechanism of action and include alkylating agents, antimetabolites, topoisomerase inhibitors, antibiotics, mitotic inhibitors, and protein kinase inhibitors. Chemotherapy is associated with a range of adverse effects (e.g., nausea, vomiting, immunosuppression, and impaired growth of healthy cells), and some agents increase the risk of secondary neoplasm development. For some chemotherapeutic agents, specific detoxifying agents can be administered to avert preventable side effects (e.g., leucovorin after application of methotrexate, mesna after cyclophosphamide application).
For further information of management chemotherapy-related complications, see “Principles of cancer care.”
Basics of chemotherapeutic agents action [1][2]
-
Kinetics
-
Chemotherapeutic agents are most active on cells with a high growth fraction, i.e., cells actively undergoing division (including normal cells, such as epithelial or bone marrow cells, as well as cancer cells)
- The log-kill hypothesis is a mathematical model of chemotherapeutic agent action, according to which a given dose of a certain chemotherapeutic agent eliminates a constant fraction of cancer cells regardless of tumor size. [3]
-
Cell cycle specificity
-
Cell cycle-specific antineoplastic agents act on proliferating cells only during a specific phase of the cell cycle. There is no cell-cycle specific antineoplastic agent that acts during the resting (G0) phase.
-
Cell cycle-nonspecific antineoplastic agents act on cells at any phase of the cell cycle, including the resting (G0) phase.
-
Resistance mechanisms: cancer cells can develop resistance to chemotherapeutic agents via the following mechanisms
- Mutations or altered expression of target cells (e.g., increased expression of dihydrofolate reductase can result in resistance of cancer cells to methotrexate)
- Increased rate of DNA repair (e.g., this mechanism can cause resistance to alkylating agents)
- Drug inactivation (e.g., some cancer cells can increase the synthesis of glutathione and other antioxidants, thus counteracting anthracyclines, which act through the generation of reactive oxygen species)
- Alteration of apoptotic pathways (e.g., leukemic cells can increase the expression of antiapoptotic molecules such as Bcl-2 to escape chemotherapy-induced apoptosis)
- Drug efflux (e.g., cancer cells can increase the expression of the MDR1 gene coding for P-glycoprotein which acts as the efflux transporter)
Basics of chemotherapy
For more information, see “Antineoplastic therapy” in “General oncology.”
Combination therapy
Chemotherapeutic agents are usually used in combination (combined chemotherapy regimens).
-
Advantages
- Increased log-kill
- Prevention and counteraction of cancer drug resistance
- Targeting both dividing and resting cells (in combination of cell cycle-specific and cell cycle-nonspecific agents)
- Synergistic effects allow for lower doses and, subsequently, less toxicity
-
Examples
-
CHOP (or R-CHOP) for the treatment of non-Hodgkin lymphomas
-
ABVD for the treatment of Hodgkin lymphomas
- FOLFOX, FOLFIRI, or XELOX for the treatment of colorectal cancer.
Routes of administration
The most common route of administration for chemotherapy is intravenous; other important methods of delivery include oral, intrathecal, and topical application.
-
Topical
- Used in the treatment of cancerous or precancerous skin lesions
- Chemotherapeutic agents that can be administered topically include 5-FU and mitomycin.
-
Intrathecal administration
- The aim of intrathecal administration is to prevent the need for cerebral radiation therapy and to treat meningeal disseminated diseases (e.g., meningeal leukemia/lymphoma)
- Chemotherapeutic agents that can be injected intrathecally include methotrexate and cytarabine.
-
Oral administration
- Allows for ambulatory treatment, e.g., maintenance therapy, palliative treatment
- Chemotherapeutic agents that can be administered orally include idarubicin, capecitabine, temozolomide, etoposide, methotrexate, 6-MP.
Efficacy of treatment
-
Individual influencing factors
- Patient factors (e.g., overall health, bone marrow capacity, liver and kidney function, age, compliance)
- Cancer factors (e.g., growth fraction, cancer doubling time, type and stage of cancer, resistance)
-
Blood-brain barrier
- Many chemotherapeutic agents cannot cross the blood-brain barrier, which reduces their effectiveness in the treatment of malignant CNS diseases
-
Fat-soluble agents (e.g., carmustine, lomustine), however, can be transported across the blood-brain barrier via diffusion and are, therefore, used in the treatment of brain tumors.
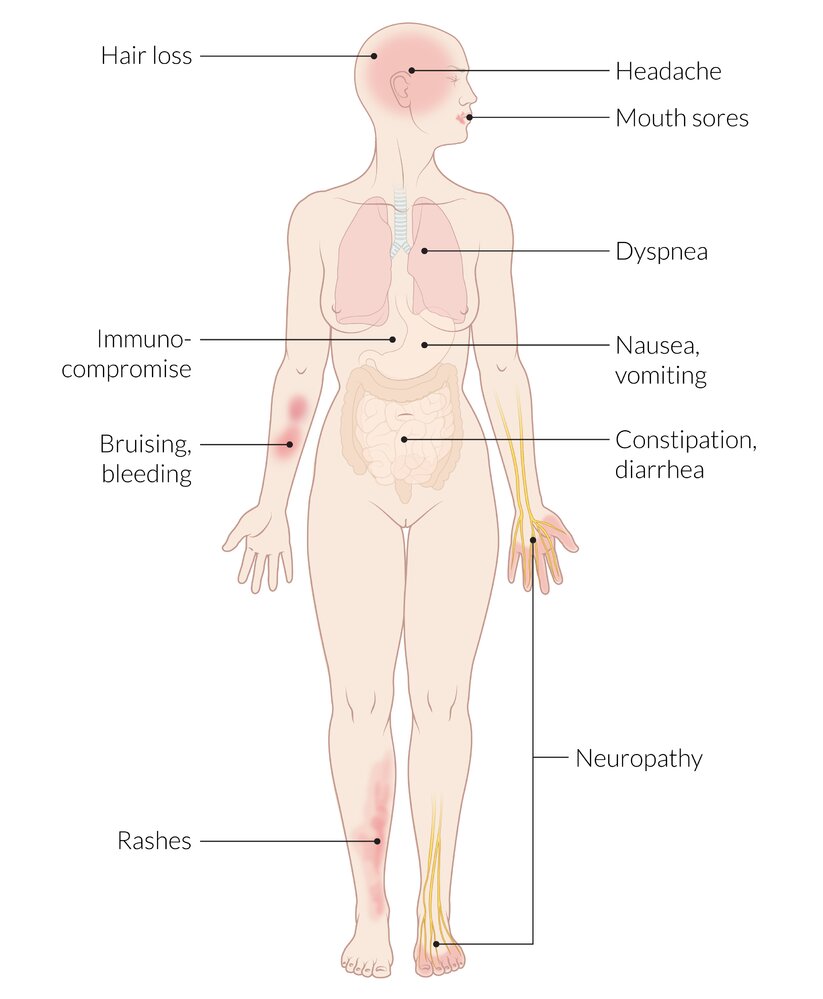
Common adverse effects of chemotherapy
Chemotherapeutic agents damage actively dividing cells, but can also affect tissues with a low mitotic potential (e.g., neurons).
Gastrointestinal tract
- Chemotherapy-induced nausea and vomiting
- Chemotherapy-induced diarrhea
-
Mucositis (soft tissue erythema of the buccal mucosa, gingival bleeding, multiple shallow ulcerations, and dysphagia)
- Constipation
- Intestinal perforation
- Causative agents include:
-
Alkylating agents (e.g., chlorambucil)
-
Antifolates (e.g., methotrexate)
-
Pyrimidine antagonists (e.g., 5-FU, cytarabine)
-
Antibiotics (e.g., dactinomycin)
-
Anthracyclines (e.g., doxorubicin, daunorubicin)
Blood
-
Myelosuppression
-
Granulocytopenia and lymphocytopenia (increased risk of infection)
-
Thrombocytopenia (increased risk of bleeding)
-
Anemia (fatigue)
- Most chemotherapeutic agents induce some extent of dose-dependent myelosuppression.
- Severe suppression of the hematopoietic system can be caused by:
-
Alkylating agents (e.g., cyclophosphamide, busulfan)
-
Antifolates (e.g., methotrexate)
-
Pyrimidine antagonists (e.g., 5-FU, cytarabine)
-
Purine antagonists (e.g., 6-MP), taxanes (e.g., paclitaxel)
-
Anthracyclines (e.g., doxorubicin, daunorubicin)
Skin
- Hair loss
- Causative agents include:
-
Pyrimidine antagonists (e.g., 5-FU)
-
Antibiotics (e.g., dactinomycin)
-
Anthracyclines (e.g., doxorubicin, daunorubicin)
CNS
-
Centrally induced vomiting
- Mediated by substance-P and neurokinin-1 receptors in the brain
- Associated with delayed emesis after chemotherapy
- Causative agents include e.g., nitrogen mustards (e.g., chlorambucil), nitrosoureas (e.g., carmustine)
-
Chemotherapy-induced peripheral neuropathy
-
Pain, burning, tingling, and loss of sensation in the distal extremities that spread from the hands and feet (stocking-glove pattern).
- Causative agents include:
-
Platinum-based medications (e.g., cisplatin)
-
Taxanes (e.g., paclitaxel)
-
Vinca alkaloids (e.g., vincristine)
Sexual organs
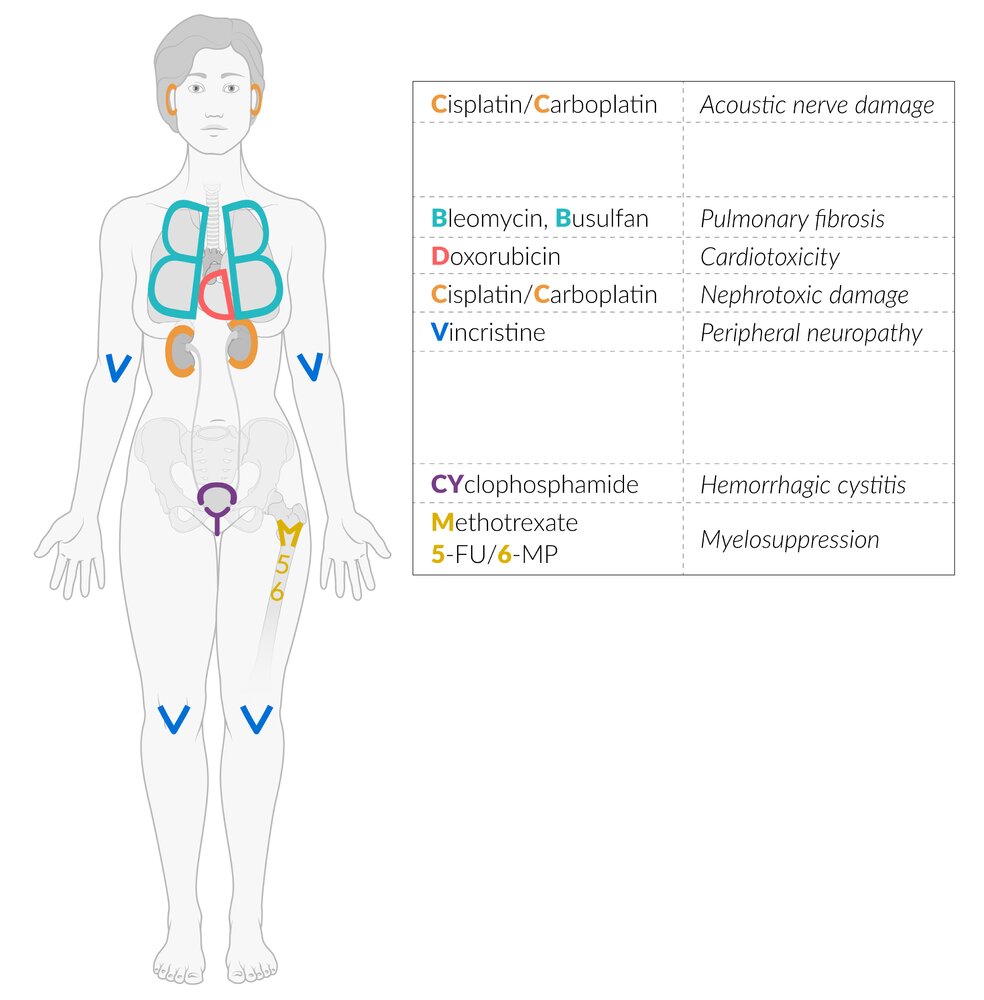
Overview of chemotherapeutic drugs classes
| Overview of important chemotherapeutic agents |
|---|
| Drug class |
Subgroup |
Drug |
Cell cycle specificity |
Indications |
| Antimetabolites |
|
|
|
-
Neoplastic conditions
- Breast cancer
-
Head and neck cancers (e.g., squamous cell carcinoma)
- Lung cancer
- Leukemias (e.g., acute lymphoblastic leukemia)
- Lymphomas (e.g., cutaneous T-cell lymphoma, non-Hodgkin lymphomas)
- Sarcomas
- Choriocarcinoma
- Other uses
- Hydatidiform moles
- Ectopic pregnancy
- Medical abortion (in combination with misoprostol)
- Immunosuppression for autoimmune diseases (e.g., rheumatoid arthritis, inflammatory bowel disease, psoriasis, vasculitides)
|
|
- Pleural mesothelioma
-
Non-small cell lung cancer (NSCLC)
|
|
- Cytarabine (arabinofuranosyl cytidine)
|
- Leukemias (e.g., AML)
- Lymphomas
|
-
5-Fluorouracil (5-FU)
-
Capecitabine (prodrug for 5-FU)
|
- Systemic treatment
- Breast cancer
- Gastric cancer
- Colorectal cancer
- Pancreatic cancer
-
Topical treatment
- Actinic keratosis
- Basal cell carcinoma
|
|
- Pancreatic cancer
- Breast cancer
- NSCLC
- Ovarian cancer
|
|
-
6-Mercaptopurine (6-MP)
- Azathioprine (prodrug for 6-MP)
|
- Acute lymphoblastic leukemia
-
Immunosuppression
- Organ transplants
- Autoimmune diseases (e.g., inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis) that are steroid-resistant or to reduce steroid dose
|
|
- Chronic lymphocytic leukemia (CLL)
-
Myeloablation prior to hematopoietic stem cell transplant
|
|
|
- Hairy cell leukemia
- Multiple sclerosis
|
- Ribonucleotide reductase inhibitors
|
-
Hydroxyurea (hydroxycarbamide)
|
|
- Myeloproliferative disorders (e.g., chronic myeloid leukemia, polycythemia vera)
- Sickle cell crisis prophylaxis (by increasing hemoglobin F)
- Leukostasis syndrome
|
| Alkylating agents |
|
|
|
-
Neoplastic conditions
-
Solid tumors (e.g., breast cancer, ovarian cancer, small cell lung cancer)
- Leukemias
- Lymphomas
- Multiple myeloma
- Nonneoplastic conditions
- Autoimmune diseases (e.g., systemic lupus erythematosus, granulomatosis with polyangiitis)
- Nephrotic syndrome
|
|
-
Solid tumors (e.g., testicular germ-cell cancer, osteosarcoma)
|
|
|
- CLL
- Hodgkin lymphoma
-
Non-Hodgkin Lymphoma (NHL)
|
|
- Multiple myeloma
- Ovarian cancer
- Amyloidosis
|
|
|
- Glioblastoma
- Anaplastic astrocytoma
|
|
- Carmustine
- Lomustine
- Streptozocin
|
- Brain tumors (e.g., glioblastoma multiforme)
-
Multiple myeloma (carmustine)
- Lymphomas
-
Pancreatic neuroendocrine tumors (streptozocin)
|
|
|
- Myeloablation prior to hematopoietic stem cell transplant
|
|
|
- Hodgkin lymphoma
-
Brain tumors (e.g., gliomas)
|
|
- Cisplatin
- Carboplatin
- Oxaliplatin
|
- Bladder cancer
- Testicular cancer
- Ovarian cancer
- Cervical cancer
- Colorectal cancer
- Lung cancer
- Osteosarcoma
|
| Topoisomerase inhibitors |
- Topoisomerase I inhibitors
|
|
|
|
|
- Cervical cancer
- Ovarian cancer
- Small-cell lung cancer (SCLC)
|
- Topoisomerase II inhibitors
|
|
- Testicular cancer
- SCLC
- Leukemias
- Lymphomas
|
|
- Leukemias (e.g., acute lymphocytic leukemia)
|
| Mitotic inhibitors |
|
|
|
-
Solid tumors
- Neuroblastoma
- Rhabdomyosarcoma
- Nephroblastoma
- Others
- Acute lymphoblastic leukemia (ALL)
- Hodgkin lymphomas and NHL
|
|
-
Solid tumors
- Kaposi sarcoma
- Langerhans cell histiocytosis
- Testicular cancer
- Others: Hodgkin lymphomas and NHL
|
|
|
|
|
- M phase
- Late G2 mitotic phase
|
- Breast cancer
- Ovarian cancer
- Prostate cancer
- Gastric cancer
- Kaposi sarcoma
- NSCLC
|
- Nontaxane microtubule inhibitors
|
|
|
- Breast cancer
- Liposarcoma
|
|
|
|
| Antibiotics |
|
|
- Squamous cell carcinomas of the head and neck
- Testicular cancer
- Hodgkin lymphoma
- Malignant pleural effusion
|
|
|
-
Childhood tumors
- Nephroblastoma
- Ewing sarcoma
- Rhabdomyosarcoma
- Gestational trophoblastic neoplasia
|
-
Anthracyclines
- Doxorubicin
- Daunorubicin
- Idarubicin
|
|
- Solid tumors
- Leukemias
- Lymphomas (Hodgkin and non-hodgkin lymphomas)
|
|
|
-
Palliative chemotherapy of gastric and pancreatic cancer
- Bladder cancer
|
|
Protein kinase inhibitors (e.g., tyrosine kinase inhibitors) |
- BCR-ABL tyrosine kinase inhibitors and c-KIT tyrosine kinase inhibitors
|
- Imatinib
- Dasatinib
- Nilotinib
|
|
- Chronic myeloid leukemia (CML) (BCR-ABL)
- ALL
- Gastrointestinal stromal tumor (c-KIT)
- Aggressive systemic mastocytosis
- Dermatofibrosarcoma protuberans
- Chronic eosinophilic leukemia
|
|
- EGFR tyrosine kinase inhibitors
|
- Erlotinib
- Gefitinib
- Afatinib
- Osimertinib
|
|
- ALK tyrosine kinase inhibitors
|
|
|
|
|
- V600E mutated-BRAF oncogene inhibitor
|
- Dabrafenib
- Vemurafenib
- Encorafenib
|
- Metastatic melanoma
- NSCLC
- Thyroid cancer
- Erdheim-Chester disease
|
|
|
|
|
- Bruton tyrosine kinase inhibitors
|
|
|
-
Neoplastic conditions
- CLL
- Mantle cell lymphoma
- Waldenstrom macroglobulinemia
- Nonneoplastic conditions: Graft-versus-host disease
|
|
|
- Polycythemia vera
- Myelofibrosis
|
|
|
|
|
| Other |
|
|
|
|
|
- Bortezomib
- Carfilzomib
- Ixazomib
|
|
- Mantle cell lymphoma (bortezomib)
- Multiple myeloma
|
|
|
|
- Breast cancer
- Ovarian cancer
- Prostate cancer
- Pancreatic cancer
|
| Monoclonal antibodies |
- See “Biological agents used in immunotherapy” in “Immunosuppressants.”
|
| Overview of important antimetabolites |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Adverse effects |
| Antifolates |
|
-
Competitive inhibition of dihydrofolate reductase via displacement of dihydrofolate → ↓ formation of pyrimidine nucleotides (↓ dTMP) and purine nucleotides → ↓ DNA synthesis
-
Inhibition of AICAR transformylase → inhibition of adenosine deaminase → ↑ intracellular concentration of adenosine and adenine nucleotides
|
-
Neoplastic conditions
- Leukemias (especially ALL)
- Lymphomas (e.g., cutaneous T-cell lymphoma, non-Hodgkin lymphomas)
- Sarcomas
- Choriocarcinoma
- Breast cancer
-
Head and neck cancers (e.g., squamous cell carcinoma)
- Lung cancer
-
Nonneoplastic conditions
- Hydatidiform moles
- Ectopic pregnancy
- Medical abortion (in combination with misoprostol)
-
Immunosuppression for autoimmune diseases (e.g., rheumatoid arthritis, inflammatory bowel disease, psoriasis, vasculitides)
|
- Myelosuppression, anemia
- Hepatotoxicity, hepatic fibrosis
- Pulmonary fibrosis, pneumonitis
- Nephrotoxicity
- Mucositis (e.g., oral ulcerations)
- Megaloblastic anemia
- Birth defects (due to folate deficiency), e.g., neural tube defects
-
Neurotoxicity (e.g., seizures)
|
|
- Multitargeted antifolate
- Inhibition of thymidylate synthase → ↓ synthesis of deoxythymidine monophosphate (dTMP) → ↓ DNA and RNA synthesis
|
- Pleural mesothelioma
- NSCLC
- Ovarian cancer
|
- Alopecia
-
Erythematous, pruritic rash (pemetrexed)
- Desquamation
- Anemia
- Pharyngitis
- GI symptoms (e.g, diarrhea)
|
| Pyrimidine antagonists |
|
- Incorporation of pyrimidine analog into DNA→ ↓ DNA synthesis (via termination of DNA chain)
- Inhibits DNA polymerase at higher doses
|
- Leukemias (especially AML)
- Lymphomas
|
-
Myelosuppression (pancytopenia)
- Megaloblastic anemia
- Hepatotoxicity
- Pancreatitis
- Sudden respiratory distress syndrome
-
Neurotoxicity (e.g., seizures, cerebellar toxicity)
|
- 5-Fluorouracil (5-FU)
- Capecitabine (prodrug for 5-FU)
|
-
Activation of 5-fluorouracil to 5-FdUMP
-
Complex formation with thymidylate synthase and folic acid → inhibition of
thymidylate synthase → ↓ dTMP production → ↓ DNA synthesis
-
Incorporation of pyrimidine analog into DNA and RNA → ↓ DNA and RNA synthesis
- Leucovorin enhances antineoplastic efficacy of 5-fluorouracil
|
- Systemic treatment
- Breast cancer
- Gastric cancer
- Colorectal cancer
- Pancreatic cancer
-
Topical treatment
- Actinic keratosis
- Basal cell carcinoma
|
- Myelosuppression
- Palmar-plantar erythrodysesthesia (hand-foot syndrome)
- Cardiotoxicity
- GI symptoms (e.g., nausea, diarrhea, mucosal ulcerations)
- Higher toxicity in patients with dihydropyrimidine dehydrogenase deficiency
- Hepatotoxicity
-
Hyperammonemic encephalopathy
|
|
-
Incorporation of pyrimidine analog into DNA → ↓ DNA synthesis
|
- Breast cancer
- NSCLC
- Ovarian cancer
- Pancreatic cancer
|
- Myelosuppression
-
Capillary leak syndrome
- Hemolytic uremic syndrome
- Pulmonary toxicity
- Hepatotoxicity
|
| Purine antagonists |
- 6-Mercaptopurine (6-MP)
- Azathioprine (prodrug for 6-MP)
|
- 6-Mercaptopurine is converted into the active metabolite by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) → ↓ de novo synthesis of purines
- Incorporation of purine analog (thiol analog) into DNA → ↓ DNA synthesis
|
- Acute lymphoblastic leukemia
-
Non-neoplastic conditions: immunosuppression
- Prevention of organ transplant rejection
-
Treatment of autoimmune diseases
- For example, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis
- Used in patients with steroid-resistance or to reduce steroid dose
|
- Myelosuppression
- GI symptoms (e.g., CINV, diarrhea)
- Hepatotoxicity
- Secondary malignancy [4]
-
Metabolized by xanthine oxidase; therefore, toxicity increases with concurrent use of allopurinol and/or febuxostat
|
|
-
Incorporation of purine analog into DNA → ↓ DNA and RNA synthesis
|
- CLL
-
Low-grade lymphomas (e.g., follicular B-cell lymphoma)
-
Myeloablation prior to hematopoietic stem cell transplant
|
- Autoimmune effects (e.g., autoimmune hemolytic anemia, idiopathic thrombocytopenia)
- Myelosuppression
- Neurotoxicity
|
|
-
Incorporation of purine analog into DNA → breakage of DNA strand → ↓ DNA synthesis
- Inhibits DNA polymerase
-
Selectively toxic to lymphocytes and monocytes that have a high deoxycytidine kinase and a low deoxynucleotidase content.
- Deoxycytidine kinase phosphorylates cladribine
-
Monophosphorylated cladribine is resistant to adenosine deaminase and accumulates within the cells. [5]
|
- Hairy cell leukemia
- CLL
-
Low-grade lymphomas
-
Nonneoplastic conditions: multiple sclerosis
|
- Myelosuppression
- Headache
- Nephrotoxicity
- Neurotoxicity
- Cardiotoxicity
- Hepatotoxicity
|
| Ribonucleotide reductase inhibitors |
-
Hydroxyurea (hydroxycarbamide)
|
- Inhibition of ribonucleotide reductase → ↓ DNA replication (S phase) → massive cytoreduction
|
-
Myeloproliferative disorders
- Chronic myeloid leukemia
- Polycythemia vera
- Essential thrombocythemia
- Leukostasis syndrome
- Head and neck cancer
|
- Myelosuppression
-
Macrocytosis, macrocytic anemia
- Secondary malignancy
- Birth defects
- Pulmonary toxicity
|
- Increases production of hemoglobin F (HbF)
|
- Sickle cell crisis prophylaxis
|
Cytarabine causes myelosuppression with pancytopenia.
| Overview of important alkylating agents |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Adverse effects |
| Oxazaphosphorines |
|
-
Alkylation of DNA/RNA →cross-linksDNA at guanine N–7 →↓ DNA replication
- Cyclophosphamide and ifosfamide require activation in the liver.
|
- Malignancies [6]
-
Solid tumors (e.g., breast cancer, ovarian cancer, small cell lung cancer)
- Leukemias
- Lymphomas
- Multiple myeloma
- Nonneoplastic conditions
- Autoimmune diseases (e.g., systemic lupus erythematosus, granulomatosis with polyangiitis)
- Nephrotic syndrome
|
-
Bladder toxicity: metabolism of oxazaphosphorines produces the urotoxic substance acrolein
-
Hemorrhagic cystitis: inflammation of the bladder, damaging to the epithelium and blood vessels
- Bladder carcinoma
- Myelosuppression
- Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
- Pulmonary toxicity
- Cardiac toxicity
- Infertility
- Fanconi syndrome (ifosfamide)
-
Neurotoxicity (ifosfamide)
|
|
-
Solid tumors (e.g., testicular germ-cell cancer, osteosarcoma) [7]
|
| Nitrogen mustards |
|
- Chronic lymphocytic leukemia
- Hodgkin lymphoma
- Non-Hodgkin lymphoma
|
- Myelosuppression
- Oral ulcerations
- GI symptoms (e.g., CINV)
- Pulmonary fibrosis
- Infertility
|
|
- Multiple myeloma
- Ovarian cancer
- Amyloidosis
|
- Myelosuppression
- Pulmonary toxicity
- Hypokalemia
- Peripheral edema
- Secondary leukemia
|
| Imidazotetrazines |
|
- Glioblastoma
- Anaplastic astrocytoma
|
- Myelosuppression
- Neurotoxicity
- Pneumocystis pneumonia
|
| Nitrosoureas |
- Carmustine
- Lomustine
- Streptozocin
|
-
Alkylation of DNA/RNA →cross-links between DNA →↓ DNA synthesis
- Require bioactivation
- Due to their high lipophilicity, carmustine and lomustine can cross the blood-brain barrier and act in the CNS.
|
- Brain tumors (e.g., glioblastoma multiforme)
-
Multiple myeloma (carmustine, lomustine)
- Hodgkin lymphoma
-
Pancreatic neuroendocrine tumors (streptozocin)
|
- Neurotoxicity (e.g., convulsions, dizziness, ataxia)
- Myelosuppression
- Pulmonary toxicity
- Secondary leukemia
|
| Alkyl sulfonate |
|
-
Cross-links between DNAstrands →↓ DNA replication
|
- Myeloablation prior to hematopoietic stem cell transplantation
- CML
|
- Severe myelosuppression (expected effect)
- Pulmonary fibrosis
- Hyperpigmentation
-
Electrolyte imbalance
- Cardiotoxicity
- Hepatotoxicity
-
Neurotoxicity (e.g., convulsions)
|
| Hydrazines |
|
- Mechanism of action is not fully understood
- Inhibition of transmethylation of methionine into transfer RNA → ↓ DNA, RNA, and protein synthesis
- Also acts as a weak MAO inhibitor
|
- Hodgkin lymphoma
-
Brain tumors (e.g., gliomas) [8]
|
- Myelosuppression
- Pulmonary toxicity
- Secondary leukemia
- Disulfiram-like reaction
- Tyramine crisis
- Gonadal damage
|
| Platinum-based agents |
- Cisplatin
- Carboplatin
- Oxaliplatin
|
-
Cross-links between DNAstrands →↓ DNA replication
|
- Lymphomas
-
Solid tumors
-
Bladder cancer (cisplatin)
-
Testicular cancer (cisplatin)
-
Ovarian cancer (cisplatin, carboplatin)
-
Colorectal cancer (oxaliplatin)
-
Lung cancer (cisplatin, carboplatin)
- Cervical cancer (cisplatin)
- Osteosarcoma (cisplatin)
|
- Myelosuppression
-
Nephrotoxicity (may manifest as Fanconi syndrome)
- Neurotoxicity (including peripheral neuropathies)
- Ototoxicity
- CINV
|
Cyclophosphamide can cause hemorrhagic cystitis.
Busulfan and Bleomycin Block your Breath: busulfan and bleomycin cause pulmonary fibrosis.
| Overview of important topoisomerase inhibitors |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Adverse effects |
| Topoisomerase I inhibitors |
|
-
Inhibition of topoisomerase I →↓ DNAunwinding →↓ DNA replication and DNA degradation (because of ssDNA breaks)
|
- Colorectal cancer
- Small-cell lung cancer
- Pancreatic cancer
|
- Myelosuppression
- GI symptoms (e.g., diarrhea)
- Cholinergic syndrome
- Alopecia
- Pulmonary toxicity (irinotecan)
|
|
- Cervical cancer
- Ovarian cancer
- Small-cell lung cancer
|
| Topoisomerase II inhibitors |
|
-
Inhibition of topoisomerase II →↑ DNA degradation (dsDNA breaks) and ↓ DNA replication (cell cycle arrest in S and G2 phase)
|
- Solid tumors
- Testicular cancer
- Small-cell lung cancer
- Leukemias
- Lymphomas
|
- Myelosuppression
- Alopecia
- Hypotension
-
Mucositis (teniposide)
|
| Overview of important mitotic inhibitors |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Adverse effects |
| Vinca alkaloids |
|
- Binding of β-tubulin → inhibition of β-tubulin polymerization into microtubules→ prevention of mitotic spindleformation →mitotic arrest of the cell in metaphase (M-phase)
|
-
Solid tumors
- Neuroblastoma
- Rhabdomyosarcoma
- Nephroblastoma
- Other
- Acute lymphocytic leukemia
- Hodgkin lymphoma
- NHL
|
- Neurotoxicity (e.g., areflexia, peripheral neuropathy)
- Paralytic ileus, constipation
- Extravasation can cause significant irritation and/or ulceration of local tissue
- Acute bronchospasm
- Uric acid nephropathy
|
|
-
Solid tumors
- Kaposi sarcoma
- Langerhans cell histiocytosis
- Testicular cancer
- Other
|
- Myelosuppression
- Extravasation can cause significant irritation of local tissue
- Pulmonary toxicity
|
|
|
- Myelosuppression
- Hypersensitivity reactions
|
| Taxanes |
|
-
Hyperstabilization of polymerized microtubules →↓ mitotic spindles breakdown →mitotic arrest in metaphase (not proceeding to anaphase)
|
- Breast cancer
- Ovarian cancer
- Prostate cancer
- Gastric cancer
- Kaposi sarcoma
- NSCLC
|
- Myelosuppression
- Neuropathy
- Hepatotoxicity
- Hypersensitivity reactions
- Fluid retention
-
Nail changes (e.g., nail bed purpura, onycholysis, nail pigmentation, splinter hemorrhage, subungual abscess)
|
| Nontaxane microtubule inhibitors |
|
- Inhibition of mitotic spindle formation → mitotic blockage → cell cycle arrest at the G2/M phase
|
- Breast cancer
- Liposarcoma
|
- Myelosuppression
- Peripheral neuropathy
- QT prolongation
|
|
- Binding to β-tubulin → hyperstabilization of the microtubules → ↓ breakdown of mitotic spindles breakdown → mitotic arrest in metaphase
|
|
- Hypersensitivity
- Myelosuppression
- Peripheral neuropathy
|
The tax rates are stable: taxanes stabilize microtubules.
Assemblies are not permitted in the vineyard: vinca alkaloids prevent microtubule assembly.
Vincristinecrisps the nerves and vinblastine blasts the bone marrow.
| Overview of important cytotoxic antibiotics |
|---|
| Agent |
Mechanism of action |
Indications |
Side effects |
| Bleomycin |
- Induces formation of free radicals → breakage of DNAstrand →cell cycle arrest at G2 phase and M phase
|
- Squamous cell carcinomas of the head and neck
- Testicular cancer
- Hodgkin lymphoma
- Malignant pleural effusion
|
-
Bleomycin-induced lung injury
- Pulmonary fibrosis
- Bronchiolitis obliterans
- Acute pneumonitis
- ARDS
- Flagellate hyperpigmentation of the skin
- Minimal myelosuppression
- Mucositis
- Alopecia
- Idiosyncratic reaction
|
|
Actinomycin D (dactinomycin)
|
-
DNA intercalation → interference with DNA transcription → ↓ RNA synthesis
|
-
Childhood tumors
- Nephroblastoma
- Ewing sarcoma
- Rhabdomyosarcoma
- Gestational trophoblastic neoplasia
|
- Myelosuppression
- Mucocutaneous toxicity
- Nephrotoxicity
- Hepatotoxicity
|
|
Anthracyclines (doxorubicin, daunorubicin, idarubicin)
|
-
Inhibition of topoisomerase II →↑ DNA degradation (dsDNA breaks) and ↓ DNA replication
-
Formation of free radicals → breakage of DNA strands
- DNA intercalation → breakage of DNA strands and ↓ DNA replication
|
-
Breast cancer (doxorubicin)
-
Metastatic solid tumors (doxorubicin)
-
Lymphomas (doxorubicin)
-
Kaposi sarcoma (doxorubicin)
-
Leukemias (daunorubicin, idarubicin)
- Osteosarcoma
|
-
Anthracycline-induced cardiotoxicity
-
Dilated cardiomyopathy with systolic CHF
-
Dose-dependent [9]
- Myelosuppression
- Alopecia
- Urine discoloration
- Extravasation
- Infertility
|
| Mitomycin |
-
Cross-linking between DNA strands → ↓ DNA and RNA synthesis
|
-
Palliative chemotherapy of gastric and pancreatic cancer
- Bladder cancer
|
- Myelosuppression
- Hemolytic uremic syndrome
- Heart failure
- Thrombotic thrombocytopenic purpura
-
Bladder fibrosis (with intravesical administration)
- ARDS
|
Busulfan and bleomycin block your breath: Busulfan and bleomycin cause pulmonary fibrosis.
Except for bleomycin, all antitumor antibiotics are cell cycle nonspecific agents. Bleomycin is effective against cells in the G2 and M phase.
| Overview of important protein kinase inhibitors |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Side effects |
| BCR-ABL and c-KIT tyrosine kinase inhibitors |
|
- Inhibition of autophosphorylation and activation of multiple proteins by tyrosine kinases (e.g.,BCR-ABL, c-KIT)
|
- Chronic myeloid leukemia
-
BCR-ABL positive ALL
- Kit (CD117)-positive gastrointestinal stromal tumors
- Aggressive systemic mastocytosis (imatinib)
- Dermatofibrosarcoma protuberans (imatinib)
-
Hypereosinophilic syndrome (imatinib)
-
Chronic eosinophilic leukemia (imatinib)
- Myelodysplastic/Myeloproliferative diseases (imatinib)
|
- General
- Fluid retention and edema
- Myelosuppression
-
Hepatotoxicity (e.g., ↑ LFTs)
- Myalgia
- For imatinib
- Neurotoxicity
-
Bullous dermatologic reactions
- Hemorrhage
- Nephrotoxicity
- For dasatinib:
- Cardiotoxicity
-
Skin rash
- Hemorrhage
- Pulmonary arterial hypertension
- QT prolongation
|
|
|
| EGFR tyrosine kinase inhibitors |
- Erlotinib
- Gefitinib
- Afatinib
- Osimertinib
|
-
Inhibition of HER1/EGFR tyrosine kinase → blockage of intracellular phosphorylation → cell death
|
|
-
Dermatologic toxicity (e.g., rash, bullous, blistering, and exfoliating skin conditions)
- Fatigue
- GI toxicity (e.g., diarrhea)
- Hepatotoxicity
- Ocular toxicity
- Nephrotoxicity
|
|
VEGFR tyrosine kinase inhibitors [10][11]
|
- Cabozantinib
- Pazopanib
- Sunitinib
- Sorafenib
- Tivozanib
- Axitinib
- Lenvatinib
- Regorafinib
- Vandetanib
|
- Inhibition of VEGF tyrosine kinase → multimodal change to tumor microenvironment via antiangiogenic effect, effects on vessel function, and immune modulation [12]
|
-
Renal cell carcinoma (e.g., sorafenib)
-
HCC (e.g., sorafenib)
-
Thyroid cancer (e.g., sorafenib)
-
Pancreatic neuroendocrine tumor (e.g., sunitinib)
-
GIST (e.g., sunitinib, regorafinib)
|
- GI toxicity (e.g., diarrhea)
- Dermatologic toxicity (e.g., rash, hand-foot syndrome)
- Cardiac toxicity (e.g., HTN)
- Fatigue
-
Thyroid toxicity
|
| ALK tyrosine kinase inhibitors |
|
- Inhibition of the anaplastic lymphoma kinase
|
|
- GI toxicity (e.g., diarrhea)
- Fluid retention and edema
- Dermatologic toxicity (e.g., rash)
- Ocular toxicity
- Neurotoxicity
- Hepatotoxicity
|
| V600E mutated-BRAF oncogene inhibitors |
|
-
Selective inhibition of BRAF oncogene with V600E mutation → inhibition of cancer cell growth
- Often administered with MEK inhibitors (e.g., trametinib)
|
- Metastatic melanoma
- NSCLC
- Thyroid cancer
|
- General
- Dermatologic toxicity (e.g., rash)
- GI toxicity (e.g., nausea, diarrhea)
- Fatigue
- QT prolongation
- For dabrafenib and encorafenib
- Cardiomyopathy
-
Febrile reactions
- Hyperglycemia
- Venous thromboembolism
- For vemurafenib
-
Dupuytren contracture and plantar fascial fibromatosis
- Pancreatitis
|
|
- Metastatic melanoma
- Erdheim-Chester disease
|
| MEK inhibitors |
|
- Inhibition of MAP kinase signaling pathway → inhibition of cancer cell growth and induction of apoptosis
|
|
- Hepatotoxicity
- Dermatologic toxicity
- GI toxicity
|
| Bruton kinase inhibitors |
|
- Inhibition of Bruton tyrosine kinase (BTK) → growth inhibition of malignant B cells
|
-
Chronic lymphocytic leukemia (CLL)
- Mantle cell lymphoma
- Waldenstrom macroglobulinemia
- Graft-versus-host disease
|
- GI toxicity
-
Cardiotoxicity (e.g., atrial fibrillation)
- Hepatotoxicity
|
| Janus kinase inhibitors |
|
-
Inhibition of JAK1 and JAK2 kinase → reduced activation of hematopoietic progenitor cells
|
- Polycythemia vera
- Myelofibrosis
|
-
Hepatotoxicity (e.g., ↑ LFTs)
- Hematologic toxicity (e.g., thrombocytopenia, anemia)
|
| CDK inhibitors |
|
- Inhibition of cyclin-dependent kinase 4 and 6 → inhibition of cancer cell growth and induction of apoptosis
|
|
- Myelosuppression
- Pulmonary toxicity (e.g., pneumonitis)
|
| Overview of chemotherapeutic agents from other groups |
|---|
| Subgroup |
Agent |
Mechanism of action |
Indications |
Side effects |
| Enzymes |
|
- Cleavage of the amino acid L-asparagine by L-asparaginase → ↓ asparagine source for leukemic cells → cytotoxicity specific to leukemic cells
|
- Acute lymphoblastic leukemia
|
- Hepatotoxicity
- Pancreatitis
-
Hypofibrinogenemia and bleeding
- Thrombosis
- Hyperglycemia
- Allergic reactions
|
| Proteasome inhibitors |
- Bortezomib
- Carfilzomib
- Ixazomib
|
- Inhibition of ubiquitinated apoptoticprotein degradation (e.g., of p53) → arrest in G2/M → programmed cell death (apoptosis)
|
- Mantle cell lymphoma (bortezomib)
- Multiple myeloma
|
- Peripheral neuropathy
- Herpes zoster reactivation
- Hepatotoxicity
- Thrombocytopenia
- Neutropenia
- Pulmonary toxicity
- Heart failure
|
| PARP Inhibitors |
|
- Inhibition of poly (ADP-ribose) polymerase → ↓ repair of single-strand DNA breaks
|
- Breast cancer
- Ovarian cancer
- Prostate cancer
- Pancreatic cancer
|
- Myelosuppression
- Fluid retention and edema
- GI toxicity (e.g., diarrhea)
|
| Monoclonal antibodies |
- See “Biological agents used in immunotherapy.”
|
VemuRAFenib and daBRAFenib are BRAF inhibitors.
Detoxifying agents for antineoplastic treatment
The toxicity of certain chemotherapeutic agents can be prevented by the administration of particular detoxifying agents.
| Overview of important detoxifying agents for antineoplastic treatment |
|---|
| Subgroup |
Agent |
Preventable adverse effect |
Detoxifying agent |
| Antifolates |
|
- Numerous adverse effects, the most important of which include:
- Myelosuppression
- Mucositis
- Hepatotoxicity
- Pulmonary fibrosis
|
-
Leucovorin (folinic acid)
- Precursor of tetrahydrofolate
- Application 24 h after the administration of antifolates
- Increases the therapeutic efficacy of thymidylate synthase inhibitors (e.g., 5–FU)
|
| Oxazaphosphorines |
- Cyclophosphamide
- Ifosfamide
|
-
Bladder toxicity
- Hemorrhagic cystitis
- Bladder carcinoma
|
- Mesna (2-MErcaptoethaneSulfonate Na) and fluids
- The sulfate group of mesna binds toxic metabolites
|
| Platinum-based agents |
- Cisplatin
- Carboplatin
- Oxaliplatin
|
-
Nephrotoxicity (may manifest as Fanconi syndrome)
|
- Amifostine: free radical scavenger
-
IV saline: induces chloride diuresis → ↑ urine chloride concentration → ↓ cisplatin reactivity
|
| Anthracyclines |
- Doxorubicin
- Daunorubicin
- Idarubicin
|
|
-
Dexrazoxane: iron chelating agent [2]
|
Management of complications
- See “Oncologic emergencies” and “Complications of cancer therapy.”

