Renal glomeruli excrete urinary substances and excess water as an ultrafiltrate into the urine by selectively filtering the blood. Any damage to the glomeruli disrupts the filtration process and results in the appearance of blood components (proteins and red blood cells) in the urine. Glomerular damage is commonly caused by immune-mediated processes, which often lead to glomerulonephritis. Non-inflammatory causes, such as metabolic disease (e.g., diabetes, amyloidosis), can also result in significant damage to the glomeruli. The pathophysiology of glomerular diseases is complex; most patients present with either nephritic syndrome (low-level proteinuria, microhematuria, oliguria, and hypertension) or nephrotic syndrome (high-level proteinuria and generalized edema). All glomerular diseases can progress to acute or chronic renal failure. Thus, quick diagnosis and immediate initiation of therapy are required to prevent irreversible kidney damage.
Terminology of glomerular diseases
- Primary: a kidney disease specifically affecting the glomeruli (e.g., minimal change glomerulonephritis)
- Secondary: a disease affecting the glomeruli in the context of a systemic disease (e.g., lupus nephritis in SLE) or a disease affecting another organ (e.g., diabetic nephropathy)
- Diffuse: > 50% of glomeruli affected (e.g., diffuse proliferative glomerulonephritis)
- Focal: < 50% of glomeruli affected (e.g., focal segmental glomerulosclerosis)
- Global: entire glomerulus is affected
- Segmental: only part of the glomerulus is affected
- Proliferative: an increased number of cells in the glomerulus
- Membranous: thickening of the glomerular basement membrane (e.g., membranous nephropathy)
- Sclerosing: scarring of the glomerulus
- Necrotizing: cell death within the glomerulus
- Crescentic: accumulation of cells such as macrophages, fibroblasts, and epithelial cells in Bowman space
- The glomerular filtration barrier consists of 3 parts
- Initial segment: Fenestrated glomerular capillary endothelium prevents large proteins from passing through.
- Second segment: The glomerular basement membrane (GBM) contains a negative charge produced by heparan sulfate.
- Final segment: Visceral epithelial cells produce/maintain the GBM and contain intercellular junctions created by podocytes that prevent further protein loss.
-
Damage to the glomeruli → disruption of the glomerular filtration barrier → can lead to nephritic or nephrotic syndrome
- See “Pathophysiology” in “Nephritic syndrome.“
- See “Pathophysiology” in “Nephrotic syndrome.“

Nephrotic vs. nephritic syndrome [1][2]
- Nephritic syndrome and nephrotic syndrome are both common clinical manifestations of glomerular diseases.
- Both syndromes are composed of characteristic clinical (e.g., edema, hypertension) and laboratory findings (e.g., glomerular hematuria, massive proteinuria), which result from damage to the glomeruli.
- Glomerular diseases are usually categorized by the syndrome they cause, which is either nephritic or nephrotic.
| Nephritic syndrome | Nephrotic syndrome | |
|---|---|---|
| Presentation |
|
|
| Pathophysiology |
|
|
| Causes |
|
|
All glomerular diseases can lead to acute and chronic kidney failure.
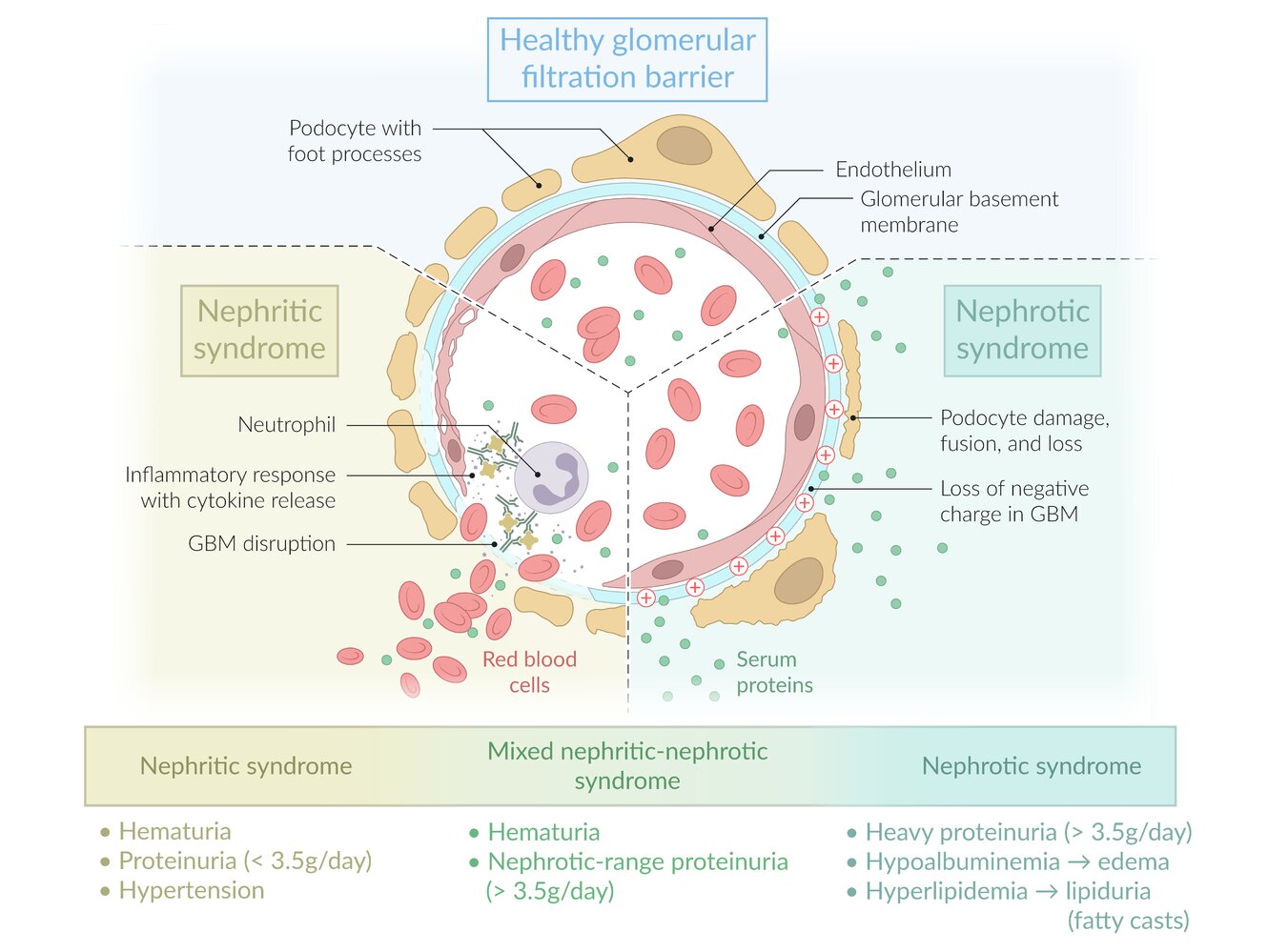
Nephritic-nephrotic syndrome
- Some diseases that manifest with nephritic syndrome can simultaneously cause nephrotic-range proteinuria (> 3.5 g/day), the main feature of nephrotic syndrome.
- When the criteria for both syndromes are fulfilled, the findings are referred to as mixed nephritic-nephrotic syndrome.
- Most common causes of nephritic-nephrotic syndrome:
- Membranoproliferative glomerulonephritis
- Diffuse proliferative glomerulonephritis
- Classifying the patient's presentation as nephritic, nephrotic, or mixed nephritic-nephrotic can help narrow down the list of likely differential diagnoses.
MPGN is a histopathological pattern of glomerular injury with various causes that is characterized by splitting of the GBM (double-contour or tram-track appearance) on light microscopy. [3]
| Overview of membranoproliferative glomerulonephritis [3][4][5] | |||
|---|---|---|---|
| Immunoglobulin (Ig)-mediated MPGN | Complement-mediated MPGN | ||
| Pathophysiology |
|
|
|
| Etiology |
|
|
|
| Clinical features |
|
||
| Laboratory studies |
|
||
| Biopsy findings | LM |
|
|
| EM [3] |
|
||
| Management [3] |
|
||
| LM = light microscopy, EM = electron microscopy | |||
MPGN is characterized by deposition of antibodies and/or complement factors in the mesangium and along capillary walls.

-
Definition
- A histopathological pattern of glomerular injury characterized by increased cellularity in > 50% of the glomeruli
- Most common and severe manifestation of lupus nephritis in systemic lupus erythematosus (SLE)
- Also seen in IgA nephropathy and in other inflammatory, autoimmune, and infectious diseases
-
Clinical features
- Nephritic syndrome
- Nephritic-nephrotic syndrome (i.e., nephritic with nephrotic-range proteinuria)
- Can lead to immune complex-mediated RPGN
-
Diagnostics
-
Laboratory studies
- ↓ Serum C3 complement levels
- In lupus nephritis: positive ANA, anti-dsDNA antibodies
- Microscopy
-
LM
- Thickening of glomerular capillaries (resembling wire loops)
- Characterized by increased cellularity in more than half of the glomeruli
- IM: granular appearance
-
EM
- More commonly: subendothelial immune deposits (IgG immune complexes, C3, and C1q)
- Less commonly: subepithelial or intramembranous deposits
-
LM
-
Laboratory studies
- Management: depends on underlying cause

| Overview of inherited glomerular disorders | |||
|---|---|---|---|
| Alport syndrome [6] | Thin basement membrane disease (benign familial hematuria) [7] | ||
| Epidemiology |
|
|
|
| Pathophysiology |
|
|
|
| Distinguishing features |
|
|
|
| Laboratory studies |
|
|
|
| Renal biopsy | LM |
|
|
| IM |
|
|
|
| EM |
|
|
|
| Management |
|
|
|